Drug Delivery 2018
Theme: Research and Implementation of Drug Delivery System
Drug Delivery 2018 proudly announces the Global Experts Meeting on World Drug Delivery and Novel Therapy Summit which will be held in Toronto, Canada. The theme of conference is “Research and Implementation of Drug Delivery System”. The conference will provide a global platform for pharmacologist, biotechnologist, medical and healthcare professionals to exchange ideas, knowledge and networking. Drug Delivery 2018 focuses on the importance to understand drugs and how they can affect human physiology. It is with better understanding of pharmacology one can know the right dosage and dosage forms of drugs.
Session 1. Drug Design and Drug Formulation:
Drug Delivery 2018 focuses on Drug Design and Drug Formulation. Pharmaceutical formula, in pharmaceutics, is the method in which different chemical materials, along with the lively drug, are combined to provide a very last medicinal product. Pre-formulation involves the characterization of a drug's physical, chemical, and mechanical houses so that you can pick what other ingredients (excipients) must be used in the coaching. In dealing with protein pre-formulation, the vital thing is to apprehend the solution behavior of a given protein below a spread of strain conditions including freeze/thaw, temperature, shear strain among others to perceive mechanisms of decay and consequently its mitigation. system research then do not forget such elements as particle length, polymorphism, pH, and solubility, as all of those can have an effect on bioavailability and therefore the hobby of a drug. The drug must be mixed with inactive substances by using a method which guarantees that the quantity of drug gift is steady in every dosage unit e.g. every tablet. The dosage need to have a uniform look, with an appropriate flavor, tablet hardness, or capsule disintegration.
Related Societies:
American Association of Pharmaceutical Scientists (AAPS), Asia; Nanotechnology Community, Asia; American Academy of Nano Medicine, USA; American Chemical Society-Nanotechnology Safety Resources, USA. British Society for Nanomedicine, USA; European Society for Biomaterials,UAE; Royal Pharmaceutical Society of Great Britain, UK.
Related Conferences:
Applied Nanotechnology and Nanoscience International Conference (ANNIC 2018), October 22 – 24,2018, Berlin (DE); International Conference On Nanomedicine And Nanobiotechnology (ICONAN 2018), September 26 – 28, 2018, Rome (IT); 18th World Congress of Basic and Clinical Pharmacology (WCP2018), July 01 – 06, 2018, Kyoto (JP); Drug Discovery Chemistry, April 02 – 06, 2018, San Diego (US).
Drug Delivery Conference | Pharmaceutical Conference | Novel Drug Delivery Conference | Drug Delivery Toronto Conference.
Session 2. Drug Targeting:
The therapeutic response of a drug depends upon the interaction of drug molecules with cell on cell membrane related biological events at receptor sites in concentration dependent manner. It is the selective and effective localization of the pharmacologically active moiety at pre-identified target in therapeutic concentration, while restricting its access to non-target normal cellular linings, thus minimizing toxic effects and maximizing the therapeutic index.
Related Societies:
International Association of Nanotechnology, USA; Microscopy Society of America, USA; American Bar Association Section Nanotechnology Project, USA; American Chemical Society-Nanotechnology Safety Resources, USA. British Society for Nanomedicine, UK; European Society for Biomaterials, UAE; Royal Pharmaceutical Society of Great Britain, USA.
Related Conferences:
Symposium on Emulsion Polymerization and Functional Polymeric Microspheres (ASEPFPM 6), March 07 – 10, 2018, Fukui (JP); 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018, Pennsylvania, USA; ICPNDDS 2018 : 20th International Conference on Pharmaceutics and Novel Drug Delivery Systems, May 17 - 18, 2018, Paris, France; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018, Prague, Czech Republic
Drug Delivery Conference | Pharmaceutical Conference | Novel Drug Delivery Conference | Drug Delivery Toronto Conference.
Session 3. Drug Delivery System and Nano Technology:
This Drug Delivery conference focuses on drug delivery whch is the method or process of administering a pharmaceutical compound to achieve a therapeutic effect in humans or animals. Drug delivery is often approached via a drug's chemical formulation, but it may also involve medical devices or drug-device combination products. Drug delivery technologies modify drug release profile, absorption, distribution and elimination for the benefit of improving product efficacy and safety, as well as patient convenience and compliance. Most common routes of administration include the preferred non-invasive peroral (through the mouth), topical (skin), transmucosal (nasal, buccal/sublingual, vaginal, ocular and rectal) and inhalation routes. Many medications such as peptide and protein, antibody, vaccine and gene based drugs, in general may not be delivered using these routes because they might be susceptible to enzymatic degradation or can not be absorbed into the systemic circulation efficiently due to molecular size and charge issues to be therapeutically effective. For this reason many protein and peptide drugs have to be delivered by injection or a nanoneedle array.
Related Societies:
American Association of Pharmaceutical Scientists (AAPS), Asia; Nanotechnology Community, Asia; American Academy of Nano Medicine, USA; American Chemical Society-Nanotechnology Safety Resources, USA. British Society for Nanomedicine, USA; European Society for Biomaterials,UAE; Royal Pharmaceutical Society of Great Britain, UK.
Related Conferences:
Applied Nanotechnology and Nanoscience International Conference (ANNIC 2018), October 22 – 24,2018, Berlin (DE); International Conference On Nanomedicine And Nanobiotechnology (ICONAN 2018), September 26 – 28, 2018, Rome (IT); 18th World Congress of Basic and Clinical Pharmacology (WCP2018), July 01 – 06, 2018, Kyoto (JP); Drug Discovery Chemistry, April 02 – 06, 2018, San Diego (US).
Drug Delivery Conference | Pharmaceutical Conference | Novel Drug Delivery Conference | Drug Delivery Toronto Conference.
Session 4. Vaccine Drug Delivery System:
Vaccine is Biological Preparation which improves immune to particular diseases. Vaccine is a material that induces an immunologically mediated resistance to a disease but not necessarily an infection. Vaccines are generally composed of killed or attenuated organisms or subunits of organisms or DNA encoding antigenic proteins of pathogens. Sub-unit vaccines though exceptionally selective and specific in reacting with antibodies often fail to show such reactions in circumstances such as shifts in epitomic identification center of antibody and are poorly immunogenic. Vaccines are the preparations given to patients to evoke immune responses leading to the production of antibodies (humoral) or cell-mediated responses that will combat infectious agents or noninfectious conditions such as malignancies. Further, surface engineering of these carriers with ligands, functional moieties and monoclonal antibodies tend to enhance the immune recognition potential of vaccines by differentiation of antigen specific memory T-cells.
Related Societies:
International Association of Nanotechnology, USA; Microscopy Society of America, USA; American Bar Association Section Nanotechnology Project, USA; American Chemical Society-Nanotechnology Safety Resources, USA. British Society for Nanomedicine, UK; European Society for Biomaterials, UAE; Royal Pharmaceutical Society of Great Britain, USA.
Related Conferences:
Symposium on Emulsion Polymerization and Functional Polymeric Microspheres (ASEPFPM 6), March 07 – 10, 2018, Fukui (JP); 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018, Pennsylvania, USA; ICPNDDS 2018 : 20th International Conference on Pharmaceutics and Novel Drug Delivery Systems, May 17 - 18, 2018, Paris, France; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018, Prague, Czech Republic
Drug Delivery Conference | Pharmaceutical Conference | Novel Drug Delivery Conference | Drug Delivery Toronto Conference.
Session 5. Smart Drug Delivery Technology:
Smart drug delivery is based on neutral phospholipid Nano liposomes. Classic liposomes modalities have had manufacturing problems involving sizing, uniformity, loading, storage, and enhancement compatibility, which can be overcome by employing true nanotechnology to build liposomes upon discrete self-assembling DNA scaffolds. The smart drug delivery system is used for delivering drugs to the host. Biological information detected by biological sensors is analyzed and the drug delivery system is actuated to deliver the drug based on the information. MEMS or NEMS technology based drug pumps, micro-pumps, micro-needles, micro-osmotic pumps, and nano-pumps are utilized for smarter drug delivery. One of the concerns these days about self-assembling nanotechnology is that it is so advanced beyond the current drug paradigm that it becomes problematic from a regulatory point of view. While there is currently no drug treatment delivered directly into these types of cancers.
Related Societies:
American Association of Pharmaceutical Scientists (AAPS), Asia; Nanotechnology Community, Asia; American Academy of Nano Medicine, USA; American Chemical Society-Nanotechnology Safety Resources, USA. British Society for Nanomedicine, USA; European Society for Biomaterials,UAE; Royal Pharmaceutical Society of Great Britain, UK.
Related Conferences:
Applied Nanotechnology and Nanoscience International Conference (ANNIC 2018), October 22 – 24,2018, Berlin (DE); International Conference On Nanomedicine And Nanobiotechnology (ICONAN 2018), September 26 – 28, 2018, Rome (IT); 18th World Congress of Basic and Clinical Pharmacology (WCP2018), July 01 – 06, 2018, Kyoto (JP); Drug Discovery Chemistry, April 02 – 06, 2018, San Diego (US).
Drug Delivery Conference | Pharmaceutical Conference | Novel Drug Delivery Conference | Drug Delivery Toronto Conference.
Session 6. Ocular Drug Delivery:
Ocular administration of drug is needed to treat ophthalmic diseases. Conventional ophthalmic formulations like solution, suspension, and ointment have many disadvantages which result into poor bioavailability of drug in the ocular cavity. The need to design a therapeutic system is to achieve an optimal concentration of a drug at the active site for a specific period of time. Various approaches have been attempted to increase the bioavailability and the duration of the therapeutic action of ocular drugs and can be divided into two categories. The first one is based on the use of sustained drug delivery systems, which provide the controlled and continuous delivery of ophthalmic drugs. The second involves maximizing corneal drug absorption and minimizing pre-corneal drug loss. The best way to optimize the drug delivery is by the addition of polymers of various grades, development of in situ gel or colloidal suspension or using erodible or non-erodible insert to prolong the pre corneal drug retention.
Related Societies:
International Association of Nanotechnology, USA; Microscopy Society of America, USA; American Bar Association Section Nanotechnology Project, USA; American Chemical Society-Nanotechnology Safety Resources, USA. British Society for Nanomedicine, UK; European Society for Biomaterials, UAE; Royal Pharmaceutical Society of Great Britain, USA.
Related Conferences:
Symposium on Emulsion Polymerization and Functional Polymeric Microspheres (ASEPFPM 6), March 07 – 10, 2018, Fukui (JP); 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018, Pennsylvania, USA; ICPNDDS 2018 : 20th International Conference on Pharmaceutics and Novel Drug Delivery Systems, May 17 - 18, 2018, Paris, France; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018, Prague, Czech Republic.
Drug Delivery Conference | Pharmaceutical Conference | Novel Drug Delivery Conference | Drug Delivery Toronto Conference.
Session 7. Anti Cancer Drug Discovery:
Anticancer drug, additionally known as antineoplastic drug, any drug that stays effective within the remedy of malignant, or cancerous, disease. There are several main instructions of anticancer capsules; those include alkylating agents, antimetabolites, herbal products, and hormones. In addition, there are some of drugs that do not fall within those instructions but that demonstrate anticancer interest and therefore are used inside the treatment of malignant sickness
Chemotherapeutic drugs
Cytotoxic and focused treatment options
Immunotherapy
treatment techniques of most cancers
surgical procedure
Radiotherapy
Oncology
Related Societies:
American Association of Pharmaceutical Scientists (AAPS), Asia; Nanotechnology Community, Asia; American Academy of Nano Medicine, USA; American Chemical Society-Nanotechnology Safety Resources, USA. British Society for Nanomedicine, USA; European Society for Biomaterials,UAE; Royal Pharmaceutical Society of Great Britain, UK.
Related Conferences:
Applied Nanotechnology and Nanoscience International Conference (ANNIC 2018), October 22 – 24,2018, Berlin (DE); International Conference On Nanomedicine And Nanobiotechnology (ICONAN 2018), September 26 – 28, 2018, Rome (IT); 18th World Congress of Basic and Clinical Pharmacology (WCP2018), July 01 – 06, 2018, Kyoto (JP); Drug Discovery Chemistry, April 02 – 06, 2018, San Diego (US).
Drug Delivery Conference | Pharmaceutical Conference | Novel Drug Delivery Conference | Drug Delivery Toronto Conference.
Session 8. Pharmacovigilance:
Pharmacovigilance (PV or PhV), additionally known as drug safety, is the pharmacological technology referring to the gathering, detection, assessment, tracking, and prevention of damaging consequences with pharmaceutical products. Pharmacovigilance closely makes a speciality of unfavorable drug reactions, or ADRs, that are described as any response to a drug that's noxious and unintentional, including lack of efficacy (the situation that this definition best applies with the doses usually used for the prophylaxis, diagnosis or therapy of disease, or for the modification of physiological disorder function changed into excluded with the brand new modification of the relevant law.
Related Societies:
International Association of Nanotechnology, USA; Microscopy Society of America, USA; American Bar Association Section Nanotechnology Project, USA; American Chemical Society-Nanotechnology Safety Resources, USA. British Society for Nanomedicine, UK; European Society for Biomaterials, UAE; Royal Pharmaceutical Society of Great Britain, USA.
Related Conferences:
Symposium on Emulsion Polymerization and Functional Polymeric Microspheres (ASEPFPM 6), March 07 – 10, 2018, Fukui (JP); 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018, Pennsylvania, USA; ICPNDDS 2018 : 20th International Conference on Pharmaceutics and Novel Drug Delivery Systems, May 17 - 18, 2018, Paris, France; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018, Prague, Czech Republic
Drug Delivery Conference | Pharmaceutical Conference | Novel Drug Delivery Conference | Drug Delivery Toronto Conference.
Session 9. Pharmacetical Regulatory Affairs:
Regulatory Affairs ensures the Quality, Safety and Efficacy of drugs. Drug development to commercialization is highly regulated. Every drug before entering into market must undergoes rigorous scrutiny and clinical trials to ensure its safety, efficacy and quality. These standards are set by regulatory authorities of their respective countries. Regulatory Affairs takes care of Development plan, supervising-writing / reviewing and assembling and submission management.
Related Societies:
American Association of Pharmaceutical Scientists (AAPS), Asia; Nanotechnology Community, Asia; American Academy of Nano Medicine, USA; American Chemical Society-Nanotechnology Safety Resources, USA. British Society for Nanomedicine, USA; European Society for Biomaterials,UAE; Royal Pharmaceutical Society of Great Britain, UK.
Related Conferences:
Applied Nanotechnology and Nanoscience International Conference (ANNIC 2018), October 22 – 24,2018, Berlin (DE); International Conference On Nanomedicine And Nanobiotechnology (ICONAN 2018), September 26 – 28, 2018, Rome (IT); 18th World Congress of Basic and Clinical Pharmacology (WCP2018), July 01 – 06, 2018, Kyoto (JP); Drug Discovery Chemistry, April 02 – 06, 2018, San Diego (US).
Drug Delivery Conference | Pharmaceutical Conference | Novel Drug Delivery Conference | Drug Delivery Toronto Conference.
Session 10. Pharmaceutical Research and Development:
Drug Delivery 2018 focuses on the recent pharmaceutical researches. Discovery process includes the primary phases of research, which are designed to categorize an investigational drug and perform primary tests in the lab. This first stage of the process takes approximately three to six years. By the end, researchers hope to identify a promising drug aspirant to further study in the lab and in animal models, and then in people. In revelation process incorporates the early periods of research, which are intended to recognize a drug and perform essential tests in the lab. This initially phase of the procedure takes around three to six years. Before the end, scientists plan to distinguish a promising medication competitor to additionally think about in the lab and in creature models, and afterward in individuals. These advances offer extraordinary guarantee, yet additionally add unpredictability to the Research and development process. With a specific end goal to guarantee the wellbeing and viability of customized treatment that are utilized close by diagnostics, clinical trial conventions must be adjusted and upgraded. This may require the utilization of extra systems and assets, and in addition new or creative types of information accumulation. In addition, by their extremely nature, the patient populace distinguished to react to focused treatments is smaller, which makes tolerant enrolment more troublesome.
Related Societies:
International Association of Nanotechnology, USA; Microscopy Society of America, USA; American Bar Association Section Nanotechnology Project, USA; American Chemical Society-Nanotechnology Safety Resources, USA. British Society for Nanomedicine, UK; European Society for Biomaterials, UAE; Royal Pharmaceutical Society of Great Britain, USA.
Related Conferences:
Symposium on Emulsion Polymerization and Functional Polymeric Microspheres (ASEPFPM 6), March 07 – 10, 2018, Fukui (JP); 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018, Pennsylvania, USA; ICPNDDS 2018 : 20th International Conference on Pharmaceutics and Novel Drug Delivery Systems, May 17 - 18, 2018, Paris, France; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018, Prague, Czech Republic.
Drug Delivery Conference | Pharmaceutical Conference | Novel Drug Delivery Conference | Drug Delivery Toronto Conference.
Summary:
The growth of biopharma, patient compliance issues and ever increasing regulatory requirements have encouraged us to adopt a drug delivery mind set from discovery through to production.This report analyzes the global markets for "Transdermal Drug Delivery System". The drug delivery technology is highly competitive market, comprising of various players. Prominent players in the drug delivery technology market include Johnson & Johnson, Inc. (U.S.), F. Hoffman-La Roche (Switzerland), Merck & Co., Inc. (U.S.), Bayer AG (Germany), Pfizer, Inc. (U.S.), Novartis AG (Switzerland), 3M Company (U.S.), Becton, Dickinson and Company (U.S.), GlaxoSmithKline plc, (U.K.), Sanofi (France), and Antares Pharma, Inc. (U.S.).The market assessment is performed through standard and the tailored research methodology approach. The report also analyzes the market by discussing market dynamics such as drivers, constraints, opportunities, threats, challenges and other market trends. The efficacious nature of these devices can be attributed to the heightened drug diffusion capabilities over conventional routes of drug delivery, namely oral, intravenous, and pulmonary. Additionally, a significant contributor of the market growth is the increasing inclination of patients as well as physicians toward pain-free drug delivery, which is further presumed to drive the market demand over the forecast period. These aforementioned factors are expected to widen the scope for the growth throughout the forecast period. But also because they realize economies of scope by sustaining diverse portfolios of research projects that capture internal and external knowledge spillovers. In pharmaceuticals, economies of scope in research are important in shaping the boundaries of the firm, and it may be worth tolerating the static efficiency loss attributable to the market power of large firms in exchange for their superior innovative performance. The global market for drug delivery systems in 2010 was $131.6 billion and is expected to increase to $137.8 billion by the end of 2011. The market is expected to rise at a compound annual growth rate (CAGR) of 5% and reach nearly $175.6 billion by 2016.
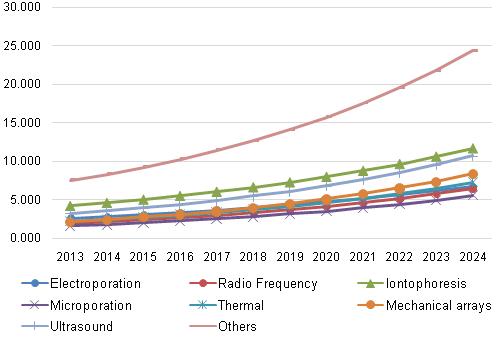
Transdermal Drug Delivery System Market, by Technology, 2013 - 2024 (USD Billion)

Important Scope:
International Journal of Drug Development and Research (IJDDR) publishes full length research reports, review articles,and scientific commentaries and communication on all aspects of the pharmaceutical sciences with strong sciences with strong emphasis on novelty, originality and scientific quality. The Editors welcome articles in this multidisciplinary field, ranging from Drug Development to Drug Discovery. BCC analyzes each market by its category with its applications in different regions of the world. It also analyzes the market leaders, the regulatory environment, technology involved, market projections, and market share. Technological issues include the latest trends and developments. Clinical Pharmacology in Drug Development is focused on publishing high-quality clinical pharmacology studies in drug development which are primarily performed in early development phases in healthy subjects. The drug delivery technology market is segmented based on route of administration, facility of use, and region. On the basis of route of administration, the market is segmented into oral, injectable, topical, nasal, ocular, pulmonary, implantable, and transmucosa. The topical drug delivery segment is expected to register the highest CAGR during the forecast period. The high growth in this segment can primarily be attributed to the factors such as convenience and ease of use, ease of dosage, painlessness, noninvasiveness, and enhanced patient compliance.
Why Canada:
Canada's ability to conduct independent research is a decisive factor for boosting nation’s competitiveness. A place for conducting good research for Drug Delivery with having institutes like Toronto Institute of Science and Technology, McGill Institute of Science and Technology, Advanced Medical Research Centers, National Institutes for Basic Biology and many more centers will come in handy. With of 1.575 GNP, Canada is unique in their science and the availability of raw materials to industrial companies offers them excellent research opportunities.
Major Drug Delivery Related Associations around the Globe
-
Pharmaceutics & Novel Drug Delivery Systems
-
Nanomedicine and Drug Delivery
-
Biopharma
-
Herbal Medicine
-
Parenteral Drug Association (PDA)
-
Medicinal Chemistry and Targeted Drug Delivery
-
Managing the Drug Discovery Process
-
New strategy for drug discovery by large-scale association analysis
-
Semantic Breakthrough in Drug Discovery
-
governing regulatory bodies across the globe
Glance at Market of Drug Discovery:
The Global Drug Delivery And Formulation Summit, organized by the WTG Events will take place from 12th to the 14th March 2018 at the Maritim proArte Hotel in Berlin, Germany.The conference will cover areas like Small Molecule Formulation & Delivery- Successful formulation strategies in achieving novel product development, Improving bioavailability of poorly soluble compounds, Biologic Formulation & Delivery- Characterisation of biological drug products, The global market for Business Development of Drug Delivery Technology in 2010 was $131.6 billion and is expected to rise at a compound annual growth rate (CAGR) of 5% and reach nearly $175.6 billion by 2016. The U.S. constituted approximately 59% of the total drug delivery market in 2010 and was $78 billion. It is forecast to reach nearly $103 billion in 2016 at a CAGR of 4.7%. Europe contributed about 27% of the total drug delivery market in 2010 and was $36 billion and is expected to grow to $49 billion by 2016 at a CAGR of 5.6% for 2013, Drug Delivery Global market reached $150.3 billion, according to BCC research. This was an increase from $142 billion the previous year.
Given its predicted annual growth the market represents a considerable business opportunity, which has been reflected in the increasing number of drug delivery specialists. Preventing aggregation and ensuring stability in biologic formulation, Emerging Technologies- Applications of nanotechnology and particle size reduction in combating issues of bioavailability, Maximising drug release control with polymer technologies, Regulations and Compliance- Harmonisation of regulations across the continent, Regulatory viewpoint on QbD in pharmaceutical development, Out-sourcing and In-licensing- Successfully sourcing new technologies from both academia and the industry, Medical Device Development- Balancing device technology enhancement with patient usability, Patient & technical considerations for device design related issues.
Conclusion:
One of the most significant drivers of the needle-free drug delivery market is the rise in the number of biopharmaceuticals. Biopharmaceuticals have shown great promise in treating various diseases and are considered standard of care in some cases. However, most biologics are administered by injection. Needle-free administration provides a cost-effective, easy to use, safe method to administer biologics without the need for traditional injection apparatus or trained personnel.
References:
http://www.nejm.org/doi/full/10.1056/NEJMp1109094#t=article
https://www.conference-service.com/conferences/pharmacology.html
http://www.competitionbureau.gc.ca/eic/site/cb-bc.nsf/eng/03631.html
https://10times.com/global-drug-delivery-and-formulation-summit
- Drug Design and Drug Formulation
- Drug Targeting
- Drug Delivery System and Nano Technology
- Vaccine Drug Delivery System
- Smart Drug Delivery Technology
- Ocular Drug Delivery
- Anti Cancer Drug Discovery
- Pharmacovigilance
- Pharmaceutical Regulatory Affairs
- Pharmaceutical Research and Development
- Journal of Pharmaceutics & Drug Delivery Research
- Journal of Pharmaceutical Sciences & Emerging Drugs
9 Organizing Committee Members
12 Renowned Speakers
Jean-Paul Lellouche
Institute of Nanotechnology and Advanced Materials (BINA)
Israel
Shereen Aly
MD Pharma Consulting Group
Canada
Bassem Toeama
University of Toronto
Canada
Oara Neumann
Rice University
USA
Zafar Iqbal
Glatt Air Techniques Inc.
USA
D S Gonzalez
Genentech
USA
Imran Vhora
Florida A&M University
USA
Deepa Patel
Parul University
India
Rikki Waterhouse
Modulation Therapeutics
UK
Guriqbal Singh
Biolyse Pharma
Canada
Neha Chavan
Glatt Air Techniques Inc.
USA
Ramandeep Kaur
RegWeb Consulting Services Inc.
Canada