
BABE 2018
Theme: BA_BE : Essential Innovation in Public and Medical Health
Meetings International proudly announces the “World Congress on Bioavailability & Bioequivalence: BA/BE Studies Summit” which will be held during August 06-07, 2018 at Tokyo, Japan The theme of conference is “BA_BE : Essential Innovation in Public and Medical Health”. Meetings International provides a Global Platform for Pharmacologist, Healthcare Professionals, Bioinformatics, Clinical and Cellular Pharmacologists and Biotech Professionals to Exchange Ideas, Knowledge and Networking at its World Conferences.
BABE 2018 mainly focuses on Bioavailability & Bioequivalence for pharmacopoeia, in the hands of clinical investigators, provide a dynamic and powerful approach to understanding the spectrum of drug development with obvious applications in Drug discovery, Pharmacogenomics, Clinical trials , Pharmacokinetics and Pharmacology and Computer-aided drug design, diagnosis, and disease management. Historically, the development of perceptive and precise bio analytical approach in the 1960s and 1970s was allowed for the first time, the measurement of very low levels of drug concentrations in biological fluids. Correspondingly, pharmacokinetic profiles of drugs, describing absorption, distribution, and clearance, could be determined. Regulations related to bioavailability and bioequivalence were put into place, considering the latest advances in the science. A significant role in the discovery, development, and regulation of new drug products involves in recent research of Bioavailability and Bioequivalence. a crucial component of abbreviated new drug applications (ANDAs), leading to market access of safe, effective, and low cost in the advance studies of bioequivalence
Why to attend??
With members from around the world focused on learning about Bioavailability & Bioequivalence: BA/BE Studies and its advances; this is your best opportunity to reach the largest assemblage of participants from the Pharmaceuticals, drug developer and its allied areas. Conduct presentations, distribute information, meet with current and potential scientists, make a splash with new Development of BABE Studies, and receive name recognition at this 2-day event. World-renowned speakers, the most recent techniques, developments, and the newest updates in BABE studies are hallmarks of this conference.
Bioavailability and Bioequivalence conference | Pharmaceutics & Novel Drug Delivery Systems Conference | Drug Discovery, Designing and Development Conference | Pharmacy and Pharmaceutical Sciences Conference
Track 1: Emerging Bioavailability & Bioequivalence studies
Historically, the development of perceptive and precise bio analytical approach in the 1960s and 1970s was allowed for the first time, the measurement of very low levels of drug concentrations in biological fluids. Correspondingly, pharmacokinetic profiles of drugs, describing absorption, distribution, and clearance, could be determined. Regulations related to bioavailability and bioequivalence were put into place, considering the latest advances in the science. A significant role in the discovery, development, and regulation of new drug products involves in recent research of Bioavailability and Bioequivalence. A crucial component of abbreviated new drug applications (ANDAs), leading to market access of safe, effective, and low cost in the advance studies in bioequivalence conference
Related Conferences:
10th Annual Congress on Drug Formulation & Analytical Techniques , September 03 - 04, 2018 Dubai, UAE; Global Experts Meeting On Public Health and Nutrition, November 5-6, 2018, Dubai, UAE ; 3rd World Congress on Public Health & Nutrition, February 26-28, 2018, London, UK ; 5th World Congress on Public Health, Nutrition & Epidemiology, July 23-24, 2018, Melbourne, Australia ; Bioavailability 2018, Understanding the bioavailability of micronutrients and bioactive compounds for improved public health10-13 September 2018 | John Innes Conference Centre, Norwich, UK. 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Japan; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Japan; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 15th European Pharma Congress, May 07-09, 2018 Frankfurt, Germany; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; World Drug Delivery and Novel Therapy Summit, July 23-24, 2018, Canada ; International Conference on Synthetic Biology July 16-17, 2018, France ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; International Conference on Nutraceuticals, May 14-15, 2018, Singapore;
Related Societies and Associations:
Generic Pharmaceutical Association ; US Food and Drug Administration( FDA) , USA; WPHNA | World Public Health Nutrition Association ; European Generic medicines Association, Europe; Therapeutics Goods Administration (TGA), UAE; European Economic Area, Europe; Canadian Generic Pharmaceutical Association (CGPA),Canada ; Bioequivalence and Bioavailability forum , Japan ; FDA Office of Surveillance and Epidemiology, USA ; American Association for Clinical Chemistry (AACC), USA ; American Association of Pharmaceutical Scientists (AAPS), USA; Clinical Trials Information from National Institutes for Health (NIH), USA; National Institute of Standards and Technology (NIST),USA;
Track 2: Advances in BA&BE
Bioavailability and bioequivalence conference is a platform to have all the scientific eminent across the globe. In pharmacology, bioavailability (BA) is a subcategory of ingestion and is the part of a managed measurement of unaltered medication that achieves the systemic flow, one of the important pharmacokinetic properties of medications. Bioavailability is one of the crucial apparatuses in pharmacokinetics, as bioavailability must be considered when computing doses for non-intravenous courses of organization.
Related Conferences;
4th Drug Discovery, Designing and Development Conference June 27-28, 2018 Vancouver, Canada ; Global Experts Meeting On Public Health and Nutrition, November 5-6, 2018, Dubai, UAE ; 3rd World Congress on Public Health & Nutrition, February 26-28, 2018, London, UK ; 5th World Congress on Public Health, Nutrition & Epidemiology, July 23-24, 2018, Melbourne, Australia ; 9th World Congress on Bioavailability & Bioequivalence April 16-18, 2018 Dubai,UAE ; Pharmaceutical & Pharmacological Research Conference, March 28-29, 2018 Japan; Bioavailability 2018, Understanding the bioavailability of micronutrients and bioactive compounds for improved public health10-13 September 2018 | John Innes Conference Centre, Norwich, UK; Global Pharma Meet & Expo February 26-28, 2018 Japan; Pharmaceutics & Novel Drug Delivery Systems conference ,December 11-13, 2017 Japan; 10th Annual Congress on Drug Formulation & Analytical Techniques , September 03 - 04, 2018 Dubai, UAE; Pharmaceutical conference and expo March 21-23, 2018 Philadelphia, USA; Pharma Conference and Expo May 2-4, 2018 ; Rome, Italy; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; World Drug Delivery and Novel Therapy Summit, July 23-24, 2018, Canada ; International Conference on Synthetic Biology July 16-17, 2018, France ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; International Conference on Nutraceuticals, May 14-15, 2018, Singapore
Related Societies and Associations:
International Society of Pharmaceutical Compounding (ISPhC), USA;WPHNA | World Public Health Nutrition Association ; International Young Pharmacists' Group (YPG), USA; Parenteral Drug Association (PDA), Japan; Regulatory Affairs Professionals Society (RAPS), USA; Society for Bio molecular Sciences (SBA), USA; Society for Cell Science (SFCS), USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE
Track 3: BA/BE of Biologic & Biosimilar
Biologic & Biosimilar conference is a platform to have all the scientific eminent across the globe. The bio similar segment is one of the fastest growing segments and is likely to reach more than USD 25 billion by 2020. Despite high development costs, the bio similar segment is likely to witness rapid growth due to the rising number of off-patent biologic drugs. The growth of this sector is attributed to the positive outcomes of on-going clinical trials and the growing demand for bio similar in different therapeutic applications. The application of advanced technologies such as recombinant DNA technology, genetic engineering, and combinatorial chemistry has increased the entry of novel biopharmaceuticals in the market which will contribute to this segment’s growth over the next four years.
Related Conference:
Pharmaceutical Chemistry and Drug Design conference, September 3-5, 2018 Japan;Global Experts Meeting On Public Health and Nutrition, November 5-6, 2018, Dubai, UAE ; 3rd World Congress on Public Health & Nutrition, February 26-28, 2018, London, UK ; 5th World Congress on Public Health, Nutrition & Epidemiology, July 23-24, 2018, Melbourne, Australia; 10th Annual Congress on Drug Formulation & Analytical Techniques , September 03 - 04, 2018 Dubai, UAE; Molecular Biology and Medicine conference, August 27-28, 2018 Japan; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Japan; 2nd Generic Drugs and Biosimilars Conference December 14-15, 2017 Rome, Italy; 12th Pharmaceutical Sciences and Innovations in Pharma Industry Congress February 26- 27, 2018 London, UK; Bioavailability 2018, Understanding the bioavailability of micronutrients and bioactive compounds for improved public health 10-13 September 2018 | John Innes Conference Centre, Norwich, UK.9th World Congress on Bioavailability & Bioequivalence April 16-18, 2018 Dubai,UAE; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; World Drug Delivery and Novel Therapy Summit, July 23-24, 2018, Canada ; International Conference on Synthetic Biology July 16-17, 2018, France ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; International Conference on Nutraceuticals, May 14-15, 2018, Singapore
Related Societies and Associations:
Generic Pharmaceutical Association ; US Food and Drug Administration( FDA) , USA; WPHNA | World Public Health Nutrition Association ; European Generic medicines Association, Europe; Therapeutics Goods Administration (TGA), UAE; European Economic Area, Europe; Canadian Generic Pharmaceutical Association (CGPA),Canada ; Bioequivalence and Bioavailability forum , Japan ; FDA Office of Surveillance and Epidemiology, USA ; American Association for Clinical Chemistry (AACC), USA ; American Association of Pharmaceutical Scientists (AAPS), USA; Clinical Trials Information from National Institutes for Health (NIH), USA; National Institute of Standards and Technology (NIST), USA;
Track 4: Drug Metabolism
Drug Metabolism conference is a platform to have all the scientific eminent across the globe. Drug metabolism is the term used to describe the biotransformation of pharmaceutical substances in the body so that they can be eliminated more easily. The metabolites of some drugs are pharmacologically active and exert an effect on the body. The active metabolite of some medications is responsible for the principal action of the drug. In this case, the drug formulation is referred to as a pro drug (some chemical substances which do not produce pharmacological effects until they are chemically altered within the body). The rate of drug metabolism affects the efficacy and toxicity of the drug for patients who have very high or low metabolism rates.
Related Conferences;
10th Annual Congress on Drug Formulation & Analytical Techniques , September 03 - 04, 2018 Dubai, UAE; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; World Drug Delivery and Novel Therapy Summit, July 23-24, 2018, Canada ; International Conference on Synthetic Biology July 16-17, 2018, France ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; International Conference on Nutraceuticals, May 14-15, 2018, Singapore; Dubai International Pharmaceuticals and Technologies Conference February 27-March 1 Japan; Global Pharma Meet April 02-04, 2018, Dubai; 9th World Congress on Bioavailability & Bioequivalence April 16-18, 2018 Dubai,UAE ; 11th Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology Meeting March 19- 22 2018 Granada, Spain; 20th Pharmacy and Pharmaceutical Sciences Conference February 15 - 16, 2018 London, United Kingdom; 11th Pharmacovigilance Conference 24 January 2018 London , UK ; Bioavailability 2018, Understanding the bioavailability of micronutrients and bioactive compounds for improved public health elsevier.com 10-13 September 2018 | John Innes Conference Centre, Norwich, UK; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE;Global Experts Meeting On Public Health and Nutrition, November 5-6, 2018, Dubai, UAE ; 3rd World Congress on Public Health & Nutrition, February 26-28, 2018, London, UK ; 5th World Congress on Public Health, Nutrition & Epidemiology, July 23-24, 2018, Melbourne, Australia; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE;
Related Societies and Associations:
International Society of Pharmaceutical Compounding (ISPhC), USA; WPHNA | World Public Health Nutrition Association; International Young Pharmacists' Group (YPG), USA; Parenteral Drug Association (PDA), Japan; Regulatory Affairs Professionals Society (RAPS), USA; Society for Bio molecular Sciences (SBA),USA; Society for Cell Science (SFCS), USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE
Track 5: New Approaches to Beat Multi-Drug Resistance: Natural and QSAR-designed Antimicrobial Peptides
Bioavailability is one of the essential tools in Pharmacokinetics, as bioavailability must be considered when calculating dosages for no intravenous routes of administration. An appropriate BE method often needs to be established based on a scientific analysis of each drug product. The above was a brief overview of bioavailability study protocol with some of its specification and essential features. Bioavailability or Bioequivalence which is actually the heart and soul of therapeutic response of a drug moiety in a given drug product.
Related Conferences;
5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Japan; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Japan; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 15th European Pharma Congress, May 07-09, 2018 Frankfurt, Germany ; World Drug Delivery and Novel Therapy Summit, July 23-24, 2018, Canada ; International Conference on Synthetic Biology July 16-17, 2018, France ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; International Conference on Nutraceuticals, May 14-15, 2018, Singapore; 9th World Congress on Bioavailability & Bioequivalence April 16-18, 2018 Dubai,UAE; 10th Annual Congress on Drug Formulation & Analytical Techniques , September 03 - 04, 2018 Dubai, UAE; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; Global Experts Meeting On Public Health and Nutrition, November 5-6, 2018, Dubai, UAE ; 3rd World Congress on Public Health & Nutrition, February 26-28, 2018, London, UK ; 5th World Congress on Public Health, Nutrition & Epidemiology, July 23-24, 2018, Melbourne, Australia;
Related Societies and Associations:
Generic Pharmaceutical Association ; US Food and Drug Administration( FDA) , USA; European Generic medicines Association, Europe; Therapeutics Goods Administration (TGA), UAE; European Economic Area, Europe; Canadian Generic Pharmaceutical Association (CGPA),Canada ; Bioequivalence and Bioavailability forum , Japan ; FDA Office of Surveillance and Epidemiology, USA ; American Association for Clinical Chemistry (AACC),USA ; American Association of Pharmaceutical Scientists (AAPS), USA; Clinical Trials Information from National Institutes for Health (NIH), USA; National Institute of Standards and Technology (NIST), USA;
Track 6: Bioequivalence Protocol: In-Vitro / In-Vivo Studies
Human pharmacokinetic conference is a platform to have all the scientific eminent across the globe. Human pharmacokinetic in vivo studies are often presumed to serve as the “gold standard” to assess product bioequivalence (BE) of immediate-release (IR) solid oral dosage forms. However, when this general assumption is re-visited, it appears that in vitro studies are sometimes better than in vivo studies in assessing BE of IR solid oral dosage forms. Reduced costs are achieved through avoiding in vivo studies where BE is self-evident, where biopharmaceutical data anticipates BE, and where in vivo BE study type II error is high. Sponsors of potential in vivo human pharmacokinetic BE testing should be required to justify why in vitro data is insufficient, similar to proposed animal testing requires justification to not employ an in vitro approach.
Related Conferences;
4th Drug Discovery, Designing and Development Conference June 27-28, 2018 Vancouver, Canada ; Pharmaceutical & Pharmacological Research Conference, March 28-29, 2018 Japan; Global Pharma Meet & Expo February 26-28, 2018 Japan; Pharmaceutics & Novel Drug Delivery Systems conference ,December 11-13, 2017 Japan; 10th Annual Congress on Drug Formulation & Analytical Techniques , September 03 - 04, 2018 Dubai, UAE; Pharmaceutical conference and expo March 21-23, 2018 Philadelphia, USA; Pharma Conference and Expo May 2-4, 2018 ; Rome, Italy ; 9th World Congress on Bioavailability & Bioequivalence April 16-18, 2018 Dubai,UAE; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE;Global Experts Meeting On Public Health and Nutrition, November 5-6, 2018, Dubai, UAE ; 3rd World Congress on Public Health & Nutrition, February 26-28, 2018, London, UK ; 5th World Congress on Public Health, Nutrition & Epidemiology, July 23-24, 2018, Melbourne, Australia; World Drug Delivery and Novel Therapy Summit, July 23-24, 2018, Canada ; International Conference on Synthetic Biology July 16-17, 2018, France ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; International Conference on Nutraceuticals, May 14-15, 2018, Singapore;
Related Societies and Associations:
International Society of Pharmaceutical Compounding (ISPhC), USA; International Young Pharmacists' Group (YPG), USA; Parenteral Drug Association (PDA), Japan; Regulatory Affairs Professionals Society (RAPS), USA; Society for Bio molecular Sciences (SBA),USA; Society for Cell Science (SFCS), USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE
Track 7: Biopharmaceutics Applications & Clinical Pharmacology
Drug product conference performance is a vital aspect of drug development as it draws on interdisciplinary expertise from both pharmaceutics and pharmacokinetics disciplines. It is at the key interface that the discipline of bio pharmaceutics has emerged. The past two decades have witnessed considerable advances in bio pharmaceutics particularly with regard to bioavailability and bioequivalence, as they relate to product quality and regulatory standards of approval.
Related Conferences;
Pharmaceutical Chemistry and Drug Design conference, September 3-5, 2018 Japan; Global Experts Meeting On Public Health and Nutrition, November 5-6, 2018, Dubai, UAE ; 3rd World Congress on Public Health & Nutrition, February 26-28, 2018, London, UK ; 5th World Congress on Public Health, Nutrition & Epidemiology, July 23-24, 2018, Melbourne, Australia;9th World Congress on Bioavailability & Bioequivalence April 16-18, 2018 Dubai,UAE ; Molecular Biology and Medicine conference, August 27-28, 2018 Japan; Advanced Materials Science & Nano Technolog; conference, August 27-29, 2018 Japan; 2nd Generic Drugs and Biosimilars Conference December 14-15, 2017 Rome, Italy; 12th Pharmaceutical Sciences and Innovations in Pharma Industry Congress February 26- 27, 2018 London, UK; 10th Annual Congress on Drug Formulation & Analytical Techniques , September 03 - 04, 2018 Dubai, UAE; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; World Drug Delivery and Novel Therapy Summit, July 23-24, 2018, Canada ; International Conference on Synthetic Biology July 16-17, 2018, France ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; International Conference on Nutraceuticals, May 14-15, 2018, Singapore;
Related Societies and Associations:
Generic Pharmaceutical Association ; US Food and Drug Administration( FDA) , USA; European Generic medicines Association, Europe; Therapeutics Goods Administration (TGA), UAE; European Economic Area, Europe; Canadian Generic Pharmaceutical Association (CGPA), Canada ; Bioequivalence and Bioavailability forum , Japan ; FDA Office of Surveillance and Epidemiology, USA ; American Association for Clinical Chemistry (AACC), USA ; American Association of Pharmaceutical Scientists (AAPS), USA; Clinical Trials Information from National Institutes for Health (NIH), USA; National Institute of Standards and Technology (NIST), USA; WPHNA | World Public Health Nutrition Association ;
Track 8: Pharmaceuticals Formulation
Pharmaceuticals Formulation conference is a platform to have all the scientific eminent across the globe. Drug product formulation is at the core of Particle Sciences. Industry-wide, delivery of active pharmaceutical ingredients (APIs) has taken center stage along with the API's themselves. Focused initially on problematic new chemical entities (NCEs), delivery systems are now the go-to approach for both NCE commercialization and the refinement and repurposing of existing APIs. The biological and chemical needs generally fall into one or a combination of a small number of categories. In addition to physicochemical and physiologic goals, business concerns also drive the use of sophisticated drug delivery systems. Intellectual property, life-cycle management, cost and market differentiations are examples.
Related Conferences;
16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; Dubai International Pharmaceuticals and Technologies Conference February 27-March 1 Japan; Global Pharma Meet April 02-04, 2018, Dubai; 11th Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology Meeting March 19- 22 2018 Granada, Spain; 20th Pharmacy and Pharmaceutical Sciences Conference February 15 - 16, 2018 London, United Kingdom; 11th Pharmacovigilance Conference 24 January 2018 London , UK; 9th World Congress on Bioavailability & Bioequivalence April 16-18, 2018 Dubai,UAE; 10th Annual Congress on Drug Formulation & Analytical Techniques , September 03 - 04, 2018 Dubai, UAE; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; World Drug Delivery and Novel Therapy Summit, July 23-24, 2018, Canada ; International Conference on Synthetic Biology July 16-17, 2018, France ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; Global Experts Meeting On Public Health and Nutrition, November 5-6, 2018, Dubai, UAE ; 3rd World Congress on Public Health & Nutrition, February 26-28, 2018, London, UK ; 5th World Congress on Public Health, Nutrition & Epidemiology, July 23-24, 2018, Melbourne, Australia ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; International Conference on Nutraceuticals, May 14-15, 2018, Singapore;
Related Societies and Associations:
International Society of Pharmaceutical Compounding (ISPhC), USA; International Young Pharmacists' Group (YPG), USA; Parenteral Drug Association (PDA), Japan; Regulatory Affairs Professionals Society (RAPS), USA; Society for Bio molecular Sciences (SBA),USA; Society for Cell Science (SFCS), USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE
Track 9: Pharmacology: PK & PD approach
Pharmacodynamics and pharmacokinetics conference is a platform to have all the scientific eminent across the globe. Pharmacodynamics and pharmacokinetics are the two principal areas of pharmacology. Pharmacodynamics is the study of the molecular, biochemical, and physiological effects of drugs on cellular systems and their mechanisms of action. Pharmacokinetics focuses rather on how the body affects the drug, in terms of its absorption, metabolism, distribution and elimination. Today, pharmacologists use a variety of techniques, including genetics, molecular biology and chemistry, to explain and manipulate the pharmacological action of substances for health purposes. BA and BE frequently rely on pharmacokinetic measures such AUC to assess extent of systemic exposure and Cmax and Tmax to assess rate of systemic absorption. It has a broad scope, from the discovery of new target molecules, to the effects of drug usage in whole populations. Bioanalytical method techniques and validation plays a vital role in the evaluation and interpretation of bioequivalence, pharmacokinetics, and toxicokinetic studies. Clinical and experimental pharmacology deals with Clinical drug developments & therapeutics. Pharmacogenomics is the study of how genetic variation influences responses to drugs. This includes how genetic variants affect drug metabolism, efficacy and toxicity, with the goal of improving and personalizing drug therapy.
Related Conferences;
5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Japan; 10th Annual Congress on Drug Formulation & Analytical Techniques , September 03 - 04, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Japan; World Drug Delivery and Novel Therapy Summit, July 23-24, 2018, Canada ; International Conference on Synthetic Biology July 16-17, 2018, France ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; International Conference on Nutraceuticals, May 14-15, 2018, Singapore;16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 15th European Pharma Congress, May 07-09, 2018 Frankfurt, Germany ; 9th World Congress on Bioavailability & Bioequivalence April 16-18, 2018 Dubai,UAE; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Global Experts Meeting On Public Health and Nutrition, November 5-6, 2018, Dubai, UAE ; 3rd World Congress on Public Health & Nutrition, February 26-28, 2018, London, UK ; 5th World Congress on Public Health, Nutrition & Epidemiology, July 23-24, 2018, Melbourne, Australia; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE.
Related Societies and Associations:
Generic Pharmaceutical Association ; US Food and Drug Administration( FDA) , USA; European Generic medicines Association, Europe; Therapeutics Goods Administration (TGA), UAE; European Economic Area, Europe; Canadian Generic Pharmaceutical Association (CGPA), Canada ; Bioequivalence and Bioavailability forum , Japan ; FDA Office of Surveillance and Epidemiology, USA ; American Association for Clinical Chemistry (AACC), USA ; American Association of Pharmaceutical Scientists (AAPS), USA; Clinical Trials Information from National Institutes for Health (NIH), USA; National Institute of Standards and Technology (NIST), USA;
Track 10: Biowaivers: Criteria
Biowaivers conference is a platform to have all the scientific eminent across the globe. Biowaivers are generally provided for multiple strengths after approval of a bioequivalence study. Biowaiver is applied to a regulatory approval process when the application (dossier) is approved based on evidence of equivalence other than an invivo bioequivalence test. For solid oral dosage forms, the evidence of equivalence is determined based on an invitro dissolution profile comparison between the multisource and the comparator product. The objective of this work was to suggest the biowaivers potential of biopharmaceutical classification system which are known to increase the solubility, dissolution, oral absorption of water insoluble drugs.
Related Conferences;
4th Drug Discovery, Designing and Development Conference June 27-28, 2018 Vancouver, Canada ; Pharmaceutical & Pharmacological Research Conference, March 28-29, 2018 Japan; Global Pharma Meet & Expo February 26-28, 2018 Japan; Pharmaceutics & Novel Drug Delivery Systems conference ,December 11-13, 2017 Japan; Pharmaceutical conference and expo March 21-23, 2018 Philadelphia, USA; Pharma Conference and Expo May 2-4, 2018 ; Rome, Italy ; 9th World Congress on Bioavailability & Bioequivalence April 16-18, 2018 Dubai,UAE; 10th Annual Congress on Drug Formulation & Analytical Techniques , September 03 - 04, 2018 Dubai, UAE; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; World Drug Delivery and Novel Therapy Summit, July 23-24, 2018, Canada ; International Conference on Synthetic Biology July 16-17, 2018, France ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; Global Experts Meeting On Public Health and Nutrition, November 5-6, 2018, Dubai, UAE ; 3rd World Congress on Public Health & Nutrition, February 26-28, 2018, London, UK ; 5th World Congress on Public Health, Nutrition & Epidemiology, July 23-24, 2018, Melbourne, Australia; International Conference on Nutraceuticals, May 14-15, 2018, Singapore;
Related Societies and Associations:
International Society of Pharmaceutical Compounding (ISPhC), USA; International Young Pharmacists' Group (YPG), USA; Parenteral Drug Association (PDA), Japan; Regulatory Affairs Professionals Society (RAPS), USA; Society for Bio molecular Sciences (SBA),USA; Society for Cell Science (SFCS), USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE
Track 11: Public Health and Nutrition
Public health conference providers across the spectrum, from individual physicians to community clinics and hospitals, are realizing that treating food insecurity and poor nutrition as a health issue can lead to better health outcomes for patients, improvements in community health, and cost savings. Increasingly, non-profit hospitals are engaging in proactive work to address food insecurity and nutrition, not only among their own patients, but more broadly as a part of their “community benefit” programs to promote population health in the communities where they are located.
Related Conferences;
Pharmaceutical Chemistry and Drug Design conference, September 3-5, 2018 Japan; Molecular Biology and Medicine conference, August 27-28, 2018 Japan; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Japan; 2nd Generic Drugs and Biosimilars Conference December 14-15, 2017 Rome, Italy; 12th Pharmaceutical Sciences and Innovations in Pharma Industry Congress February 26- 27, 2018 London, UK ; 9th World Congress on Bioavailability & Bioequivalence April 16-18, 2018 Dubai,UAE; 10th Annual Congress on Drug Formulation & Analytical Techniques , September 03 - 04, 2018 Dubai, UAE; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; World Drug Delivery and Novel Therapy Summit, July 23-24, 2018, Canada ; International Conference on Synthetic Biology July 16-17, 2018, France ; Global Experts Meeting On Public Health and Nutrition, November 5-6, 2018, Dubai, UAE ; 3rd World Congress on Public Health & Nutrition, February 26-28, 2018, London, UK ; 5th World Congress on Public Health, Nutrition & Epidemiology, July 23-24, 2018, Melbourne, Australia; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; International Conference on Nutraceuticals, May 14-15, 2018, Singapore;
Related Societies and Associations:
Generic Pharmaceutical Association ; US Food and Drug Administration( FDA) , USA; European Generic medicines Association, Europe; Therapeutics Goods Administration (TGA), UAE; European Economic Area, Europe; Canadian Generic Pharmaceutical Association (CGPA), Canada ; Bioequivalence and Bioavailability forum , Japan ; FDA Office of Surveillance and Epidemiology, USA ; American Association for Clinical Chemistry (AACC), USA ; American Association of Pharmaceutical Scientists (AAPS), USA; Clinical Trials Information from National Institutes for Health (NIH), USA; National Institute of Standards and Technology (NIST), USA;
Track 12: Regulatory Requirement and Approaches
Failure at any stage would mean a huge loss for the company. Hence, a lot of planning is required even before the project is underway. Recently, with the use of technology the process is becoming a less risky business, because of the ability of the computers to predict the possible outcomes. This will surely reduce the efforts in fruitless directions. Hence, the most important and most common biological targets for drug discovery are either enzymes regulating the biochemistry or the receptors through which many hormones and endogenous effectors show their response.
Related Conferences;
9th World Congress on Bioavailability & Bioequivalence April 16-18, 2018 Dubai,UAE ; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; Dubai International Pharmaceuticals and Technologies Conference February 27-March 1 Japan; Global Pharma Meet April 02-04, 2018, Dubai; 11th Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology Meeting March 19- 22 2018 Granada, Spain; 20th Pharmacy and Pharmaceutical Sciences Conference February 15 - 16, 2018 London, United Kingdom; 11th Pharmacovigilance Conference 24 January 2018 London , UK ; 10th Annual Congress on Drug Formulation & Analytical Techniques , September 03 - 04, 2018 Dubai, UAE ; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; World Drug Delivery and Novel Therapy Summit, July 23-24, 2018, Canada ; Global Experts Meeting On Public Health and Nutrition, November 5-6, 2018, Dubai, UAE ; 3rd World Congress on Public Health & Nutrition, February 26-28, 2018, London, UK ; 5th World Congress on Public Health, Nutrition & Epidemiology, July 23-24, 2018, Melbourne, Australia; International Conference on Synthetic Biology July 16-17, 2018, France ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; International Conference on Nutraceuticals, May 14-15, 2018, Singapore;
Related Societies and Associations:
International Society of Pharmaceutical Compounding (ISPhC), USA; International Young Pharmacists' Group (YPG), USA; Parenteral Drug Association (PDA), Japan; Regulatory Affairs Professionals Society (RAPS), USA; Society for Bio molecular Sciences (SBA),USA; Society for Cell Science (SFCS), USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE;
Track 13: Pharmaceuticals Industry: Entrepreneur Meet
Entrepreneur Meet is a platform to bring all the eminent speakers and researchers together in this conference The decision to terminate a trial early due to unacceptable toxicity or substantial and convincing evidence of benefit is complex and must account for many factors These include possible imbalances in risk factors between treatment groups, whether the patients have the risk profile assumed in the design, patient compliance to therapy, quality and timeliness of data, possible sources of bias, consistency of primary and secondary outcome variables, the benefit-to-risk ratio, consistency of results with external data, and the impact of early termination on the medical community as well as the public. Evaluation of these issues goes beyond routine statistical tests and requires the collective judgment of experts, such as those represented by a DSMB. For decades the creation of new medicines has increased quality and quantity of life for patients around the globe.
Related Conferences;
Pharmaceutical Chemistry and Drug Design conference, September 3-5, 2018 Japan; 9th World Congress on Bioavailability & Bioequivalence April 16-18, 2018 Dubai,UAE; Molecular Biology and Medicine conference, August 27-28, 2018 Japan; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Japan; 2nd Generic Drugs and Biosimilars Conference December 14-15, 2017 Rome, Italy; 12th Pharmaceutical Sciences and Innovations in Pharma Industry Congress February 26- 27, 2018 London, UK ; 10th Annual Congress on Drug Formulation & Analytical Techniques , September 03 - 04, 2018 Dubai, UAE; Advanced Materials Science & Nano Technology conference, August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Global Experts Meeting On Public Health and Nutrition, November 5-6, 2018, Dubai, UAE ; 3rd World Congress on Public Health & Nutrition, February 26-28, 2018, London, UK ; 5th World Congress on Public Health, Nutrition & Epidemiology, July 23-24, 2018, Melbourne, Australia; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; World Drug Delivery and Novel Therapy Summit, July 23-24, 2018, Canada ; International Conference on Synthetic Biology July 16-17, 2018, France ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; World Congress on Applied Microbiology Rome, Italy, June 25-26, 2018 ; International Conference on Nutraceuticals, May 14-15, 2018, Singapore;
Related Societies and Associations:
Generic Pharmaceutical Association ; US Food and Drug Administration( FDA) , USA; European Generic medicines Association, Europe; Therapeutics Goods Administration (TGA), UAE; European Economic Area, Europe; Canadian Generic Pharmaceutical Association (CGPA), Canada ; Bioequivalence and Bioavailability forum , Japan ; FDA Office of Surveillance and Epidemiology, USA ; American Association for Clinical Chemistry (AACC), USA ; American Association of Pharmaceutical Scientists (AAPS), USA; Clinical Trials Information from National Institutes for Health (NIH), USA; National Institute of Standards and Technology (NIST), USA;
Introduction
The pharmaceutical industry is comprised of companies engaged in researching, developing, manufacturing and distributing drugs for human or veterinary use. New drugs have an enormous positive influence on global health, prosperity and economic productivity by saving lives, increasing life spans, reducing suffering, preventing surgeries and shortening hospital stays. Advances in medicine have eliminated deadly diseases and have brought other life-threatening conditions under control. Drug therapy is now an integral part of nearly every facet of healthcare, and new breakthroughs promise to revolutionize the treatment of non-communicable diseases.
Industry Overview and Competitiveness:
Large, diversified and global, the U.S. pharmaceutical industry is one of the most critical and competitive sectors in the economy. According to the Pharmaceutical Research and Manufacturers Association (PhRMA), more than 810,000 people work in the biopharmaceutical industry in the United States across a broad range of occupations, such as scientific research, technical support and manufacturing. Directly and indirectly, the industry supports over 3.4 million jobs across the United States and added an estimated $790 billion to the economy in 2014. Although manufacturing jobs supported by the industry are expected to decline over the next decade due to continued productivity gains, it will remain an important source of high paying jobs, providing salaries way above the national average.
Research and development (R&D)
The pharmaceutical sector has consistently been one of the most R&D intensive industries in the United States. The research-based industry generally allocates around 15 to 20 per cent of revenues to R&D activities and invests over $50 billion on R&D annually. Although the United States remains the global leader in innovative R&D investment, producing more than half the world’s new molecules in the last decade, its continued leadership cannot be taken for granted. R&D performed in the United States has become increasingly expensive relative to emerging economies in Asia, such as China and Singapore, where governments have enacted policies to attract investment and are poised for future growth. Conditions that limited R&D offshoring in the past, such as market proximity and availability of talent, are rapidly shifting.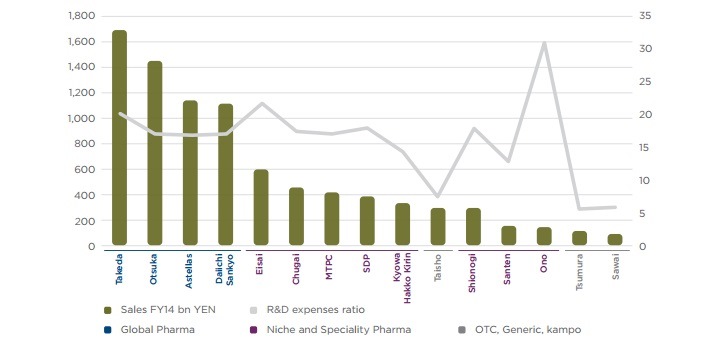
The worldwide market for pharmaceuticals is projected to grow from around $1 trillion in 2015 to $1.3 trillion by 2020, representing an annual growth rate of 4.9 per cent. Several global demographic and economic trends are driving pharmaceutical consumption, including a rapidly aging world population and an associated rise in chronic diseases, increased urbanization and higher disposable incomes, greater government expenditure on healthcare and growing demand for more effective treatments.
Societies/Industries/Universities Associated with Pharmaceutical Technology and Development:-
- International Society of Pharmaceutical Compounding (ISPhC)
- International Young Pharmacists' Group (YPG)
- Parenteral Drug Association (PDA)
- Regulatory Affairs Professionals Society (RAPS)
- Society for Bio molecular Sciences (SBA)
- Society for Cell Science (SFCS)
- Al-Hayat Pharmaceuticals
- Amgen
- Elis Pharmaceuticals Limited
- Alliance Global
- Sanofi Aventis UAE
- Genpharm
- Ranbaxy Laboratories Limited
- BioPharma
- Hokkaido University
- Waseda University
- Nagoya University
Major Pharmaceutical Associations around the Globe
- Bio analytical Focus Group and Ligand Binding Assay
- American Association of Pharmaceutical Scientists (AAPS)
- Royal Netherlands Chemical Society
- The European Bio analysis Forum
- BEBAC Consultancy Services for Bioequivalence and Bioavailability
- European Federation of Pharmaceutical Industries and Associations (EFPIA)
- European Generics and Bio similar Medicines Association (EGA)
- Canadian Generic Pharmaceutical Association (CGPA)
- Bioequivalence and Bioavailability forum
- Generic Pharmaceutical Association (GPhA)
- Pharmaceutical Management Agency (PHARMAC)
- FDA Office of Surveillance and Epidemiology
- Drug Watch
- ORPHANET Parenteral Drug Association
- PharmGKB
- US Food and Drug Administration (FDA)
- American Association for Clinical Chemistry (AACC)
- American Association of Pharmaceutical Scientists (AAPS)
- Clinical Trials Information from National Institutes for Health (NIH)
- National Institute of Standards and Technology (NIST)
- Association of the British Pharmaceutical Industry (ABPI)
- Pharmaceutical Research and Manufacturers of America (PhRMA)
- Medicines and Healthcare products Regulatory Agency (MHRA)
- Innovative Medicines Canada
Why in Japan???
Japan has a large, well-developed pharmaceutical market and offers universal health coverage to its citizens. However its traditional low-cost healthcare model is now trending towards other developed countries. In the absence of major healthcare reforms Japan will experience a funding gap of ¥19.2 trillion (~ $160 billion) by 2020 which is expected to rise to ¥44.2 trillion (~ $370 billion) by 2035. Increasing insurance premiums to fund the gap will increase labour costs and harm Japan’s competitive position. Co-payment rates are already high at 30%, and an additional increase would undermine the concept of health insurance.3–5 So, Japan needs alternative financial options.
Despite flat economic growth, Japan’s position in world pharmaceutical sales is projected to remain significant. The market in Japan is projected to grow 17% over the period 2011–2020 (based on constant exchange rates).Without a doubt, Japan Health Authority is attempting to build up the medicinal tourism part, expecting to draw in 1.3 million restorative visitors yearly by 2021. The revenue on medicinal tourism is required to develop by 13% every year for the following five years. The growth opportunities outside of Japan and the developed markets are significant for those companies who are poised to access them.
- Emerging Bioavailability & Bioequivalence Studies
- Advances in BA&BE
- BA/BE of Biologic & Biosimilar
- Drug Metabolism
- New Approaches to Beat Multi-Drug Resistance: Natural and QSAR-designed Antimicrobial Peptides
- Bioequivalence Protocol: In-Vitro / In-Vivo Studies
- Biopharmaceutics Applications & Clinical Pharmacology
- Pharmaceuticals Formulation
- Pharmacology: PK & PD approach
- Vaccines, Blood & Biologics
- Public Health and Nutrition
- Regulatory Requirement and Approaches
- Pharmaceuticals Industry: Entrepreneur Meet
- Pharmaceutical Science and Emerging Drugs
- Journal of Current chemical and pharmaceutical sciences
7 Organizing Committee Members
12 Renowned Speakers
Pawan Saharan
CEO, Biomix Network Inc. USA & Biomix Network Limited
India
Yuan Chen
Genentech
USA
Inwook Choi
Korea Food Research Institute
South Korea
Abdeen Mustafa Omer
Energy Research Institute, Nottingham
UK
Kateryna Zupanets
National University of Pharmacy
Ukraine
Viktoriia Propisnova
National University of Pharmacy
Ukraine
Yahdiana Harahap
Faculty of Pharmacy,
University Indonesia,
Indonesia
Vladyslava Chernykh
National University of Pharmacy
Ukraine
Jacob A. Kolawole
University of Jos.
Nigeria
Sepideh KHORASANI
University of Kerman
Iran
Andras Fodor
University of Pannonia
Hungary
Mohamed Fahmy Zeid
Alexandria University
Egypt

































