
Clinicaltrials-2022

Theme: Innovative Strategies for Future Expansion in Clinical Trials
International Conference on Clinical Trials is going to be held in Berlin, Germany on September 21-22, 2022 which includes prompt keynote presentations, Oral talks, Workshops/Symposia, Poster presentations, and Exhibitions. The theme of the conference is, “Innovative Strategies for Future Expansion in Clinical Trials”. Clinical Trails Summit is the leading event that brings together an eccentric and international consort of experts, researchers, and decision-makers both from academia and industry across the globe to exchange their knowledge, expertise, and research innovations to form a remarkable clinical trials conference.
Clinical Trials Conference promotes better understanding by the general public about the importance of clinical trials in the prevention, diagnosis and treatment of disease. It is the best platform to encourage the sincere administration of medication and explore the best case reports where ultimate results to justify curing and healing by various procedures to various ailments with evidence and providing the righteous option for treating ailments.
Details of Clinical Trials Conference in 2022 in Germany:
Meetingsint.com organizing Clinical Trials Conferences in 2022 in Germany and Worldwide. Our Clinical Research Meetings focus on phases and trials of drugs, devices and procedures.
| Conference Name | Place | Date |
|---|---|---|
| Clinical Trials Conferences 2022 | Berlin, Germany | September 21-22, 2022 |
Session 1: Innovations in Clinical Trials
Clinical trials for the possibilities of new remedies and their most part started and financed by industry. There are various clinical trials initiated by clinical professionals. Clinical research is frequently performed all over the world, which can now and then, be expansive ones. As an outcome, clinical research is exceedingly controlled.
Clinical Trials Events | Clinical Trials Conferences | Clinical Research Conferences | Clinical Operations Conferences
Session 2: Pre-clinical Research
Pre-clinical research is a stage of research that begins before clinical trials and during which important feasibility, iterative testing and drug safety data are collected, typically in laboratory animals. The main goals of pre-clinical research are to determine a starting, safe dose for first-in-human study and assess for the potential toxicity of the product, which typically include new medical devices, prescription drugs, and diagnostics.
Clinical Conferences | Research Events | Clinical Summit | Scientific Research conferences
Session 3: Clinical Trials on Different Diseases
Clinical Trials for different diseases leads to assessing at least one medication for treating a disorder or a condition. Discovering approaches to keep the underlying advancement or continuous ailments or conditions. These can incorporate immunizations or way of changes among various aspects.
Clinical Trials Conference | Clinical Research Meet | Clinical research Summit | Clinical Trials Meetings
Session 4: Design of Clinical Studies
Clinical study design is the formation of trials, experiments, and observational studies in medical, clinical and epidemiological. The goal of a clinical study is to assess the safety, efficacy, and the mechanism of action of an investigational medicinal procedure. It can also be to investigate a drug, device or procedure that has already been approved but is still in need of further investigation, typically with respect to long-term effects or cost-effectiveness.
Clinical Trials Events | Clinical Trials Conferences | Clinical Research Conferences | Clinical Operations Conferences
Session 5: Drug Discovery and management
In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered. A variety of approaches is employed to identify chemical compounds that may be developed and marketed. Discovering drugs may be a commercial success, or a public health success, involves a complex interaction between investors, industry, academia, patent laws, regulatory exclusivity and marketing. Meanwhile, for disorders whose rarity means that no large commercial success or public health effect can be expected, the orphan drug funding process ensures that people who experience those disorders can have some hope of pharma therapeutic advances.
Clinical Conferences | Research Events | Clinical Summit | Scientific Research conferences
Session 6: Pharmacovigilance and Drug Safety
Pharmacovigilance is the pharmacological science relates to the collection, detection, assessment, monitoring, and prevention of adverse effects with pharmaceutical products. Pharmacovigilance heavily focuses on adverse drug reactions which are defined as any response to a drug which is noxious and unintended including lack of efficacy. Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breastfeeding, are also of interest, even without an adverse event, because they may result in an adverse drug reaction.
Clinical Trials Conference | Clinical Research Meet | Clinical research Summit | Clinical Trials Meetings
Session 7: Bioethics and Regulatory Compliance
Bioethics is the investigation of the commonly questionable moral issues rising up out of new circumstances and potential outcomes realized by advances in medication. It is additionally moral insight as it identifies with therapeutic approach, practice, and research. One of the principal ranges tends to present day bioethicists were that of human experimentation.
Clinical Trials Events | Clinical Trials Conferences | Clinical Research Conferences | Clinical Operations Conferences
Session 8: Microbiology Clinical Research
Clinical microbiology research is a discipline that encompasses a broad range of testing methodologies. Although significant improvements in testing methodologies have been made, clinical microbiology remains heavily reliant on culture-based methods and phenotypic methods for identification of culture organisms. Medical microbiology is a branch of medical science concerned with the prevention, diagnosis and treatment of infectious diseases. In addition, this field of science studies various clinical applications of microbes for the improvement of health.
Clinical Conferences | Research Events | Clinical Summit | Scientific Research conferences
Session 9: Latest technologies in Clinical Research
Technology plays a critical role in drug development and the R&D value chain by revolutionizing clinical trials and decreasing the failure rate. Though the supply of technology has been increasing and regulation of innovative methods is easing, pharmaceutical companies have been slow to use the emerging technologies, due to the ambiguity prevailing around this space and a highly fragmented supply market. This article outlines the key technologies that have a high impact across trial phases.
Research Events | Clinical Summit | Scientific Research conferences | Clinical Trials Conference
Session 10: Clinical Data Management and Statistics
Clinical Data Management (CDM) is a critical phase in clinical research, which leads to generation of high-quality, reliable, and statistically sound data from clinical trials. This helps to produce a drastic reduction in time from drug development to marketing. Team members of CDM are actively involved in all stages of clinical trial right from inception to completion. With the implementation of regulatory compliant data management tools, CDM team can meet these demands.
Clinical Trials Conference | Clinical Research Meet | Clinical research Summit | Clinical Trials Meetings
Session 11: Clinical and Medical Case Reports
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports may contain a demographic profile of the patient, but usually describe an unusual or novel occurrence. Some case reports also contain a literature review of other reported cases. Case reports are professional narratives that provide feedback on clinical practice guidelines and offer a framework for early signals of effectiveness, adverse events, and cost. They can be shared for medical, scientific, or educational purposes.
Research Events | Clinical Summit | Scientific Research conferences | Clinical Trials Conference
Session 12: Stem Cell and Genetic Clinical Research
In recent years, clinical trials with stem cells have taken the emerging field in many new directions. While numerous teams continue to refine and expand the role of bone marrow and cord blood stem cells for their vanguard uses in blood and immune disorders, many others are looking to expand the uses of the various types of stem cells found in bone marrow and cord blood, in particular mesenchymal stem cells, to uses beyond those that could be corrected by replacing cells in their own lineage. Early results from these trials have produced mixed results often showing minor or transitory improvements that may be attributed to extracellular factors.
Clinical Conferences | Research Events | Clinical Summit | Scientific Research conferences
Clinical Trials 2022 aims to gather leading educational scientists, researchers and research scholars to exchange and share their experiences and research results about all aspects of research. It additionally delivers the prospect for researchers, practitioners and educators to gift and confer the foremost recent innovations, trends, and issues, sensible challenges encountered and also the solutions enforced in their related clinical research fields. We bring together industrial executives, Pharmacy and Health care sectors making the conference an impeccable platform to network, share views and knowledge through interactive discussions.
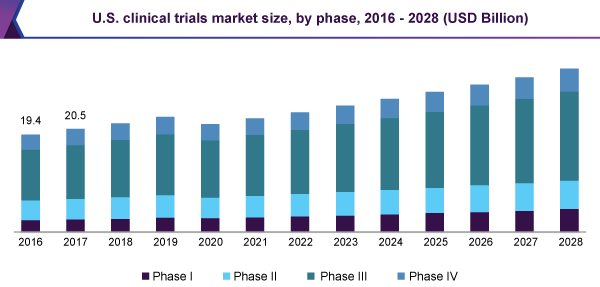
Future Scope:
The Clinical Trials statistical surveying report states that interest for Researchers will probably profit by social insurance change. The speed of progressions in clinical research has been energized by the guarantee that new discoveries may improve the consideration of patients experiencing renal sickness. Clinical Trials extent of training has qualified significant changes in the on-going decade. This development is to a great extent because of the extension of the leading perpetual tests understanding populace, advancing human services conveyance models in a time of outrageous budgetary requirement and mechanical advancement adjusting care conveyance to sick patients. So as to suit the heterogenic patient populace and the wide collection of training settings, the researcher must be set up to fill in as the patient's essential consideration supplier or potentially subspecialty expert. The utilization of cooperative practice models in consideration is basic to address the issues of this quickly extending patient populace.
Clinical Research Societies around the world:
ACM Global Laboratories
Axon
Bio Reliance Corporation
Bioclinica
Biotrial
Clinical supplies management holdings
Celerion
Charles River Laboratories
Clinical Site Services (CSS)
Clinlogix
GenScript
Harrison Clinical
ICON
IQVIA
Top Clinical Research Universities Worldwide:
Asia:
The University of Tokyo, Japan
Kyoto University, Japan
National University of Singapore, Singapore
University of Hong Kong, Hong Kong
Seoul National University, South Korea
National Taiwan University, Taiwan
Osaka University, Japan
The University of Queensland, Australia
Tsinghua University, China
The University of Tokyo, Japan
Kyoto University, Japan
National University of Singapore, Singapore
University of Hong Kong, Hong Kong
Seoul National University, South Korea
National Taiwan University, Taiwan
Osaka University, Japan
The University of Queensland, Australia
Tsinghua University, China
Europe:
Karolinska Institute, Sweden
Heidelberg University, Germany
ETH Zurich, Switzerland
Charité - Universitätsmedizin, Germany
LMU Munich, Germany
KU Leuven – University of Leuven, Belgium
Erasmus University Rotterdam, The Netherlands
Sorbonne University, France
University of Paris, France
Maastricht University, The Netherlands
University of Zurich, Switzerland
Karolinska Institute, Sweden
Heidelberg University, Germany
ETH Zurich, Switzerland
Charité - Universitätsmedizin, Germany
LMU Munich, Germany
KU Leuven – University of Leuven, Belgium
Erasmus University Rotterdam, The Netherlands
Sorbonne University, France
University of Paris, France
Maastricht University, The Netherlands
University of Zurich, Switzerland
America:
Harvard University, Massachusetts
Johns Hopkins University, Maryland
University of California, California
Stanford University, California
University of Washington, Washington
University of Pennsylvania, Philadelphia
Duke University, North Carolina
Harvard University, Massachusetts
Johns Hopkins University, Maryland
University of California, California
Stanford University, California
University of Washington, Washington
University of Pennsylvania, Philadelphia
Duke University, North Carolina
Canada:
University of Toronto, Ontario
McGill University, Montreal
University of British Columbia, Vancouver And Kelowna
Queen's University, Ontario
University of Toronto, Ontario
McGill University, Montreal
University of British Columbia, Vancouver And Kelowna
Queen's University, Ontario
Middle East:
Tel Aviv University, Israel
Hebrew University of Jerusalem, Israel
Technion Israel Institute of Technology, Israel
Weizmann Institute of Science, Israel
Ben Gurion University of the Negev, Israel
King Saud University, Saudi Arabia
University of Tehran, Iran
King Abdulaziz University, Saudi Arabia
Middle East Technical University, Turkey
King Abdullah University of Science & Technology, Saudi Arabia
Tel Aviv University, Israel
Hebrew University of Jerusalem, Israel
Technion Israel Institute of Technology, Israel
Weizmann Institute of Science, Israel
Ben Gurion University of the Negev, Israel
King Saud University, Saudi Arabia
University of Tehran, Iran
King Abdulaziz University, Saudi Arabia
Middle East Technical University, Turkey
King Abdullah University of Science & Technology, Saudi Arabia
Conclusion:
Clinical Trials 2022 will bring together experts like eminent scientists, senior/junior research fellows, professors, students, members of different medical and clinical association, medicine and medical technology companies from all over the world to share an interest in the field of clinical research to discuss about the new concepts and research ideas with developments in the field which is to be applied in near future for the prosperity of humans. Development of new techniques of diagnosis, treatment and prevention has to be recognized which will imply a significant role on the lives of people. The distributed findings and researches regarding this topic will be given another priority as these are the stepping stones for the future.
Clinical Trials 2022 will bring together experts like eminent scientists, senior/junior research fellows, professors, students, members of different medical and clinical association, medicine and medical technology companies from all over the world to share an interest in the field of clinical research to discuss about the new concepts and research ideas with developments in the field which is to be applied in near future for the prosperity of humans. Development of new techniques of diagnosis, treatment and prevention has to be recognized which will imply a significant role on the lives of people. The distributed findings and researches regarding this topic will be given another priority as these are the stepping stones for the future.
Clinical Trials 2022 helps to inform and attract new customers quickly and efficiently. The size and diversity of our advertising options, including banners, sponsored emails, article alerts or newsletters, provide clients with the very best customized marketing opportunities in science and medicine. We are one of the renowned events organizing platform with most of eminent speakers and business audience form all over the globe indexed. The advertising platform we provide you is the best chance of showcasing your products/services, and branding your company. If you are looking for a global exposure for your products and services, this is the right place for you. With over 5 million readers worldwide and nearly 3 million hits a month on our website, we have engaged audience of students, research scholars, scientists, doctors, professors, pharmacists and professionals from companies across the territories. If you sell research materials, products, medicinal instruments, or service like rehabilitation centres, here is your opportunity to advertise in the website that can connect you to leading experts and science specialists across the Globe. Advertisement banner must be provided in high resolution jpg or jpeg format by the advertising company and must not have copyright infringement. We can also support your events and conferences by providing you with high quality reprints of published articles that can add value to your event.
Meetings International is announcing the Young Scientist Awards through International Conference on Clinical Trials which is scheduled in Berlin, Germany on September 21-22, 2022. This Clinical Trials 2022 focuses on “Innovative Strategies for Future Expansion in Clinical Trials”. The clinical research meeting and upcoming conferences will recognize participants who have significantly added value to the scientific community of Medical science and provide them outstanding Young Scientist Awards. The Young Scientist Award will provide a strong professional development opportunity for young researches by meeting experts to exchange and share their experiences at our international conferences. The conference operating committee is providing a platform for all the budding young researchers, young investigators, post-graduate/Master students, PhD. students and trainees to showcase their research and innovation. Eligibility: Young Scientists, faculty members, post-doctoral fellows, PhD scholars and bright Final Year MSc and M.Phil. Candidates Persons from Scientific Industry can also participate.
Benefits:
- The Young Scientist Feature is a platform to promote young researchers in their respective area by giving them a chance to present their achievements and future perspectives
- Acknowledgement as YRF Awardee
- Promotion on the conference website, Young Scientist Awards and certificates
- Link on the conference website
- Recognition on Meetings Int. Award Page
- Chances to coordinate with partners around the world
- Research work can be published in the relevant journal without any publication fee
Criteria:
- All presented abstracts will automatically be considered for the Award
- All the presentation will be evaluated in the conference venue
- All the awards will be selected by the judges of the award category
- The winners of the Young Scientist Award will receive award certificate
- The awards will be assessed as far as plan and format, intelligence, argumentation and approach, familiarity with past work, engaging quality, message and primary concerns, parity of content visuals, and by and large impression
Guidelines:
- All submissions must be in English
- The topic must fit into scientific sessions of the conference
- Each individual participant is allowed to submit maximum 2 papers
- Abstract must be submitted online as per the given abstract template
- Abstracts must be written in Times New Roman and font size will be 12
- Abstract must contain title, name, affiliation, country, speakers biography, recent photograph, image and reference
Conditions of Acceptance:
To receive the award, the awardee must submit the presentation for which the award is given, for publication at the website, along with author permission. Failure to submit the PPT and permission within the designated timeframe will result in forfeiture of award.
Award Announcements:
Official announcement of the recipients will occur after the completion of Clinical Trials 2022.
Meetings International has taken an initiative to congratulate attendees with awards to recognize, celebrate and encourage action in its varied conferences and events. We have a tendency to salute and acknowledge attainment among business that's persistently evolving and re-drawing the boundaries of best follow. These awards represent the top of skilled action for event professionals. To win a present you'll are supported by our illustrious panel of the foremost business professionals that could be a successful form of your formidable credentials.
Best Speaker in Clinical Trials 2022:
This award identifies educational and business speakers for his or her wonderful presentation skills and for the numerous contributions that they create to their professions, their groups and their patients through their follow, leadership or analysis endeavor. Recipient of this award are chosen by the session chair and co-chair. The awardee are felicitated when the completion of oral session. Our International Conference on Clinical Trials encourages researchers from across the world to require half as a speaker at these prestigious events.
Best Key Note Speaker Clinical Trials 2022:
This award recognizes the notable shows given throughout the course of the event. It additionally acknowledges the keenness and determination of the speaker to enhance the expertise of all World Health Organization they are available into contact with. The award are given supported the impact of presentation on the attendees of the event. We'll even be taking under consideration the references taken from the citations of the keynote speaker. These awards are felicitated by one in all the members of the organizing panel gift throughout the conference.
Best Organizing Member Clinical Trials 2022:
This award pursues to recognize the continual support provided by the Organizing member of this prestigious conference. Recipient of this award are designated by the operative committee of the event. The award are given supported the support and cooperation provided by a member of the organizing panel from the start to the roaring completion of the conference.
Outstanding Young Scientists Award in Clinical Trials 2022:
This award seeks to recognize the young brains of this era. Recipient of this award are designated by choosing the Young Researchers Forum. This award are given supported the presentation skills, impact of analysis and its applications for the Clinical Trials 2022. Participants following Masters, Doctor of Philosophy or Post-Doctoral studies are eligible for this award. The awards are felicitated when the completion of all the oral shows delivered below the Young Researchers Forum class.
Best Poster Award Clinical Trials 2022:
This award recognizes the most effective poster presentation given throughout the course of an occurrence. Recipient of this award are designated by choosing the poster session. a present winning poster are evaluated on presentation content and clarity, originality of approach, communication criteria and scientific aspects. It'll even be supported layout, insights, analysis and results description. The awardee are felicitated when the completion of poster session.
The awards given for our aid conferences recognize the big selection of responsibilities and dedication of Clinical scholars World Health Organization promote the very best standards of care across the complete health service. Clinical Trials 2022 event create a large contribution to maintaining and protective the health and well-being of individuals across the world.
- Innovations in Clinical Trials
- Pre-clinical Research
- Clinical Trials on Different Diseases
- Design of Clinical Studies
- Drug Discovery and management
- Pharmacovigilance and Drug Safety
- Bioethics and Regulatory Compliance
- Microbiology Clinical Research
- Latest technologies in Clinical Research
- Clinical Data Management and Statistics
- Clinical and Medical Case Reports
- Stem Cell and Genetic Clinical Research
- Journal of Health Informatics & Management
2 Organizing Committee Members
12 Renowned Speakers
Ibrahim El Bayoumy
Professor of public health and community medicine, Tanta faculty of medicine,
Egypt
Amirova Mahira Firudin
Azerbaijan Medical University
Azerbaijan
FATIMA YOUSEF ALI GHETHAN
Quality and Medication Safety Consultant
King Abdullah Medical City
Saudi Arabia
KIARA MARIE PADUA
Makati Medical Center, Makati City,
Philippines
MIRZA MUHAMMAD FARAN ASHRAF BAIG
Faculty of Dentistry, The University of Hong Kong,
Hong Kong
DAVID BELLIDO-YARLEQUAE
Vascular Surgery Unit,
Peru
HEINZ-PETER SCHULTHEISS
Institute for Cardiac Diagnostics and Therapy, Berlin,
Germany
PRABHAT ADHIKARI
Nepal Health Research Council,
Nepal
MAHMOUD METWALY TAHA
Saudi German Hospital, Aseer,
Saudi Arabia
GUZAL TOSHMATOVA
Tashkent Medical Academy, Tashkent,
Uzbekistan
ABDUL M GBAJ
Department of Medicinal Chemistry, Faculty of Pharmacy, University of Tripoli,
Libya
ZAW THANT LWIN
Senior registrar,
Scotland



































































































































