
BABE-2019
Theme: Essential Innovation in the Field of Pharmaceutical Science for Public and Medical health
It’s an enormous pleasure and honor to organize “15th World Congress on Bioavailability and Bioequivalence” scheduled during July 29-30, 2019 at Bangkok, Thailand. The conference is mainly focused on the theme of “Essential Innovation in the field of Pharmaceutical Science for Public and Medical health”. BABE Conference 2019 is designed with prudent keynote sessions, session lectures, and poster presentations, presentations from the young researchers, panel discussions, and the BABE meetings with world-renowned speakers from the stream of clinical and pharmaceutical sciences. It provides the best platform for the researchers to the researchers all over globe to introduce themselves to the world with their unique researches. It’s an open forum to discuss new researches and the challenges faced during the BA/BE studies, manufacturing the generic drugs and their effect on the health. BABE 2019 is a 2days event offering the Exhibition, a venue to showcase the new and emerging technologies and have wider sessions involving keynote presentation, oral, young researchers forum, poster, e-poster presentations. World-renowned speakers and eminent delegates across the globe attending the conference, to share their valuable presentation on the most recent and advanced techniques, developments, and the newest updates are the prominent features of the conference.
Session 1: Advances in BA&BE
Bioavailability and bioequivalence meeting is a stage to have all the logical prominent over the globe. In pharmacology, bioavailability (BA) is a subcategory of ingestion and is the piece of an oversaw estimation of unaltered prescription that accomplishes the fundamental stream, one of the imperative pharmacokinetic properties of drugs. Bioavailability is one of the significant mechanical assemblies in pharmacokinetics, as bioavailability must be considered when figuring dosages for non-intravenous courses of association.
Keywords: Pharmaceutical Conferences | Pharmaceutical Events | Drug Delivery Events | Pharma Conferences | Pharmaceutical Meetings | Pharma Industry Meet | Pharma Exhibitions | Pharmacy Workshop | Pharma Seminar | BABE Meeting | BABE Events | Asia Pharma Conference | Asia Medicine Conferences | Europe Pharma Conference | Europe Medicine Conferences | USA Pharma Conference | USA Medicine Conferences
Related Societies and Associations:
International Society of Pharmaceutical Compounding, USA; World Public Health Nutrition Association, USA; International Young Pharmacists' Group, USA; Parenteral Drug Association, Japan; Regulatory Affairs Professionals Society, USA; Society for Bio molecular Sciences, USA; Society for Cell Science, USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE
Session 2: BA/BE of Biologic & Biosimilar
Biologic and Bio comparative meeting is a stage to have all the logical famous over the globe. The bio comparative portion is one of the quickest developing fragments and is probably going to achieve more than USD 25 billion by 2020. In spite of high advancement costs, the bio comparable fragment is probably going to observe fast development because of the rising number of off-patent biologic medications. The development of this segment is ascribed to the positive results of on-going clinical preliminaries and the developing interest for bio comparative in various remedial applications. The use of trend setting innovations, for example, recombinant DNA innovation, hereditary building, and combinatorial science has expanded the section of novel biopharmaceuticals in the market which will add to this current fragment's development throughout the following four years.
Keywords: Pharmaceutical Conferences | Pharmaceutical Events | Drug Delivery Events | Pharma Conferences | Pharmaceutical Meetings | Pharma Industry Meet | Pharma Exhibitions | Pharmacy Workshop | Pharma Seminar | BABE Meeting | BABE Events | Asia Pharma Conference | Asia Medicine Conferences | Europe Pharma Conference | Europe Medicine Conferences | USA Pharma Conference | USA Medicine Conferences
Related Societies and Associations:
International Society of Pharmaceutical Compounding, USA; World Public Health Nutrition Association, USA; International Young Pharmacists' Group, USA; Parenteral Drug Association, Japan; Regulatory Affairs Professionals Society, USA; Society for Bio molecular Sciences, USA; Society for Cell Science, USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE
Session 3: Pharmacodynamics
Pharmacodynamics (PD) is the investigation of the biochemical and physiologic impacts of medications (particularly pharmaceutical medications). The impacts can incorporate those showed inside creatures (counting people), microorganisms, or mixes of life forms (for instance, contamination). Pharmacodynamics is the investigation of how a medication influences a life form, while pharmacokinetics is the investigation of how the life form influences the medication. Both together impact dosing, advantage, and antagonistic impacts. Pharmacodynamics is once in a while curtailed as PD and pharmacokinetics as PK, particularly in joined reference (for instance, when discussing PK/PD models). Pharmacodynamics places specific accentuation on dose– reaction connections, that is, the connections between medication focus and impact.
Keywords: Pharmaceutical Conferences | Pharmaceutical Events | Drug Delivery Events | Pharma Conferences | Pharmaceutical Meetings | Pharma Industry Meet | Pharma Exhibitions | Pharmacy Workshop | Pharma Seminar | BABE Meeting | BABE Events | Asia Pharma Conference | Asia Medicine Conferences | Europe Pharma Conference | Europe Medicine Conferences | USA Pharma Conference | USA Medicine Conferences
Related Societies and Associations:
International Society of Pharmaceutical Compounding, USA; World Public Health Nutrition Association, USA; International Young Pharmacists' Group, USA; Parenteral Drug Association, Japan; Regulatory Affairs Professionals Society, USA; Society for Bio molecular Sciences, USA; Society for Cell Science, USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE
Session 4: Pharmacovigilance
Pharmacovigilance is the pharmacological science ensuring safety and related to the collection, detection, assessment, monitoring, and prevention of adverse side effects with pharmacological action of pharmaceutical products. According to US FDA a drug is regarded as safe by looking at side effects, its manufacturing process and results of animal testing and clinical trials. In this track, we discuss Drug safety and its applications in various fields, Pharmacovigilance and its Significance and Scope present to record its growth and potential as an important discipline within Medical science, and to describe its impact on patient welfare and public health and to know what is pharmacovigilance.
Keywords: Pharmaceutical Conferences | Pharmaceutical Events | Drug Delivery Events | Pharma Conferences | Pharmaceutical Meetings | Pharma Industry Meet | Pharma Exhibitions | Pharmacy Workshop | Pharma Seminar | BABE Meeting | BABE Events | Asia Pharma Conference | Asia Medicine Conferences | Europe Pharma Conference | Europe Medicine Conferences | USA Pharma Conference | USA Medicine Conferences
Related Societies and Associations:
International Society of Pharmaceutical Compounding, USA; World Public Health Nutrition Association, USA; International Young Pharmacists' Group, USA; Parenteral Drug Association, Japan; Regulatory Affairs Professionals Society, USA; Society for Bio molecular Sciences, USA; Society for Cell Science, USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE
Session 5: New Approaches to Beat Multi-Drug Resistance: Natural and QSAR-designed Antimicrobial Peptides
Bioavailability is one of the fundamental apparatuses in Pharmacokinetics, as bioavailability must be considered while ascertaining measurements for no intravenous courses of organization. A suitable BE strategy frequently should be built up in light of a logical examination of each medication item. The above was a concise review of bioavailability contemplates convention with a portion of its particular and basic highlights. Bioavailability or Bioequivalence which is really the substance of helpful reaction of a medication moiety in a given drugs products.
Keywords: Pharmaceutical Conferences | Pharmaceutical Events | Drug Delivery Events | Pharma Conferences | Pharmaceutical Meetings | Pharma Industry Meet | Pharma Exhibitions | Pharmacy Workshop | Pharma Seminar | BABE Meeting | BABE Events | Asia Pharma Conference | Asia Medicine Conferences | Europe Pharma Conference | Europe Medicine Conferences | USA Pharma Conference | USA Medicine Conferences
Related Societies and Associations:
International Society of Pharmaceutical Compounding, USA; World Public Health Nutrition Association, USA; International Young Pharmacists' Group, USA; Parenteral Drug Association, Japan; Regulatory Affairs Professionals Society, USA; Society for Bio molecular Sciences, USA; Society for Cell Science, USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE
Session 6: Bioequivalence Protocol: In-Vitro / In-Vivo Studies
Human pharmacokinetic gathering is a stage to have all the logical prominent over the globe. Human pharmacokinetic in vivo considers are regularly ventured to fill in as the "best quality level" to survey item bioequivalence (BE) of quick discharge strong oral measurements shapes. In any case, when this general suspicion is returned to, it creates the impression that in vitro ponders are here and there superior to in vivo considers in surveying BE of IR strong oral measurement frames. Decreased expenses are accomplished through maintaining a strategic distance from in vivo ponders where BE is plainly obvious, where biopharmaceutical information foresees BE, and where in vivo BE think about sort II mistake is high. Patrons of potential in vivo human pharmacokinetic BE trying ought to be required to legitimize why in vitro information is deficient, like proposed creature testing expects avocation to not utilize an in vitro approach.
Keywords: Pharmaceutical Conferences | Pharmaceutical Events | Drug Delivery Events | Pharma Conferences | Pharmaceutical Meetings | Pharma Industry Meet | Pharma Exhibitions | Pharmacy Workshop | Pharma Seminar | BABE Meeting | BABE Events | Asia Pharma Conference | Asia Medicine Conferences | Europe Pharma Conference | Europe Medicine Conferences | USA Pharma Conference | USA Medicine Conferences
Related Societies and Associations:
International Society of Pharmaceutical Compounding, USA; World Public Health Nutrition Association, USA; International Young Pharmacists' Group, USA; Parenteral Drug Association, Japan; Regulatory Affairs Professionals Society, USA; Society for Bio molecular Sciences, USA; Society for Cell Science, USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE
Session 7: Biopharmaceutics Applications & Clinical Pharmacology
Medication item gathering execution is an imperative part of medication improvement as it draws on interdisciplinary mastery from the two pharmaceutics and pharmacokinetics disciplines. It is at the key interface that the reach of bio pharmaceutics has raised. The previous two decades have seen impressive advances in bio pharmaceutics especially with respect to bioavailability and bioequivalence, as they identify with item quality and regulatory standards of approval.
Keywords: Pharmaceutical Conferences | Pharmaceutical Events | Drug Delivery Events | Pharma Conferences | Pharmaceutical Meetings | Pharma Industry Meet | Pharma Exhibitions | Pharmacy Workshop | Pharma Seminar | BABE Meeting | BABE Events | Asia Pharma Conference | Asia Medicine Conferences | Europe Pharma Conference | Europe Medicine Conferences | USA Pharma Conference | USA Medicine Conferences
Related Societies and Associations:
International Society of Pharmaceutical Compounding, USA; World Public Health Nutrition Association, USA; International Young Pharmacists' Group, USA; Parenteral Drug Association, Japan; Regulatory Affairs Professionals Society, USA; Society for Bio molecular Sciences, USA; Society for Cell Science, USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE
Session 8: Pharmacology: PK & PD approach
Pharmacodynamics and pharmacokinetics meeting is a stage to have all the logical famous over the globe. Pharmacodynamics and pharmacokinetics are the two important regions of pharmacology. Pharmacodynamics is the investigation of the atomic, biochemical, and physiological impacts of medications on cell frameworks and their components of activity. Pharmacokinetics centers rather on how the body influences the medication, as far as its retention, digestion, circulation and end. BA and BE every now and again depend on pharmacokinetic measures such AUC to evaluate degree of foundational presentation and Cmax and Tmax to survey rate of fundamental retention. It has a wide extension, from the revelation of new target particles, to the impacts of medication utilization in entire populaces. Bioanalytical strategy strategies and approval assumes an essential part in the assessment and translation of bioequivalence, pharmacokinetics, and toxicokinetic thinks about. Clinical and exploratory pharmacology manages Clinical medication improvements and therapeutics. Pharmacogenomics is the investigation of how hereditary variety impacts reactions to drugs. This includes how genetic variants affect drug metabolism, efficacy and toxicity, with the goal of improving and personalizing drug therapy.
Keywords: Pharmaceutical Conferences | Pharmaceutical Events | Drug Delivery Events | Pharma Conferences | Pharmaceutical Meetings | Pharma Industry Meet | Pharma Exhibitions | Pharmacy Workshop | Pharma Seminar | BABE Meeting | BABE Events | Asia Pharma Conference | Asia Medicine Conferences | Europe Pharma Conference | Europe Medicine Conferences | USA Pharma Conference | USA Medicine Conferences
Related Societies and Associations:
International Society of Pharmaceutical Compounding, USA; World Public Health Nutrition Association, USA; International Young Pharmacists' Group, USA; Parenteral Drug Association, Japan; Regulatory Affairs Professionals Society, USA; Society for Bio molecular Sciences, USA; Society for Cell Science, USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE
Session 9: Public Health and Nutrition
Public Health conference providers across the spectrum, from individual physicians to community clinics and hospitals, are realizing the treating food insecurity and poor nutrition as a health issue can lead to better health outcomes for patients, improvements in community health, and cost savings. Increasingly, non-profit hospitals are engaging in proactive work to address and food insecurity and nutrition, not only among their own patients, but more broadly as a part of their “community benefit” programs to promote population health in community where they are located.
Keywords: Pharmaceutical Conferences | Pharmaceutical Events | Drug Delivery Events | Pharma Conferences | Pharmaceutical Meetings | Pharma Industry Meet | Pharma Exhibitions | Pharmacy Workshop | Pharma Seminar | BABE Meeting | BABE Events | Asia Pharma Conference | Asia Medicine Conferences | Europe Pharma Conference | Europe Medicine Conferences | USA Pharma Conference | USA Medicine Conferences
Related Societies and Associations:
International Society of Pharmaceutical Compounding, USA; World Public Health Nutrition Association, USA; International Young Pharmacists' Group, USA; Parenteral Drug Association, Japan; Regulatory Affairs Professionals Society, USA; Society for Bio molecular Sciences, USA; Society for Cell Science, USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE
Session 10: Regulatory Requirement and Approaches
Failure at any stage would mean a huge loss for the company. Hence, a lot of planning is required even before the project is underway. Recently, with the use of technology the process is becoming a less risky business, because of the ability of the computers to predict the possible outcomes. Thus will surely reduce the efforts in fruitless directions. Hence, the most important and most common biological targets for drug discovery are either enzymes regulating the biochemistry or the receptors through which many hormones and endogenous effectors show their response.
Keywords: Pharmaceutical Conferences | Pharmaceutical Events | Drug Delivery Events | Pharma Conferences | Pharmaceutical Meetings | Pharma Industry Meet | Pharma Exhibitions | Pharmacy Workshop | Pharma Seminar | BABE Meeting | BABE Events | Asia Pharma Conference | Asia Medicine Conferences | Europe Pharma Conference | Europe Medicine Conferences | USA Pharma Conference | USA Medicine Conferences
Related Societies and Associations:
International Society of Pharmaceutical Compounding, USA; World Public Health Nutrition Association, USA; International Young Pharmacists' Group, USA; Parenteral Drug Association, Japan; Regulatory Affairs Professionals Society, USA; Society for Bio molecular Sciences, USA; Society for Cell Science, USA; Al-Hayat Pharmaceuticals, USA; Amgen, Europe; Elis Pharmaceuticals Limited, Japan; Alliance Global, Japan; Sanofi Aventis UAE , UAE; Genpharm, UAE; Ranbaxy Laboratories Limited, USA; BioPharma, Japan; Hokkaido University , USA; Waseda University ,UAE; Nagoya University, UAE
The Pharmaceutical industry is comprised of companies engaged in researching, developing, manufacturing and distributing drugs for human or veterinary use. New drugs have an enormous positive influence on global health, prosperity and economics productivity by saving lives, increasing life spans, reducing suffering, preventing surgeries and shortening hospital stays. Advances in medicine have eliminated deadly diseases and have brought other life threatening conditions under control. Drug therapy is now an integral part of nearly every facet of healthcare, and new breakthroughs promise to revolutionize the treatment of non-part of non-communicable disease.
Why in Thailand??
Thailand is a beautiful country, the capital is Bangkok which is contain 513,120 km2 and more than 68 million populations. Thailand is the world's 50th biggest nation by add up to region and the 21st-most-crowded nation. Pacific Bridge Medical has proficient accomplices and vital partners in Thailand who offer an assortment of business advancement and administrative administrations to help medicinal gadget and pharmaceutical organizations and huge technology in the Thai restorative markets.
Health and medical care is overseen by the Ministry of Public Health, along with total national expenditures on health amounting to 4.3 percent of GDP in 2017. Non-communicable diseases form the major burden of morbidity and mortality, while infectious diseases including malaria and tuberculosis, as well as traffic accidents, and are also important public health issues.
Tourism makes up about 6% of the economy. Thailand's attractions include hundreds of tropical islands, nightlife, archaeological sites, museums, hill tribes, flora and bird life, palaces, Buddhist temples and several World Heritage sites. Many tourists follow courses during their stay in Thailand. Popular are classes in Thai cooking, Buddhism and traditional Thai massage. Among the best-known are the "Elephant Round-up" in Surin, the "Rocket Festival" in Yasothon, Suwannaphum District, and Phanom Phrai District both district are located in Roi ET Province and the "Phi Ta Khon" festival in Dan Sai. Thai cuisine has become famous worldwide with its enthusiastic use of fresh herbs and spices. Bangkok shopping malls offer a variety of international and local brands.
Why to attend??
With members from around the world focused on learning about Bioavailability & Bioequivalence: BA/BE Studies and its advance; this is your best opportunity to reach the largest assemblage of participants from the pharmaceuticals, drug developer and its allied areas. Conduct presentations, distribute information, meet with the current potential scientists, make a splash with new development of BABE Studies, and receive name recognition at this 2-day event. World –renowned speakers, the most recent techniques, developments and the newest updates in BABE studies are hallmarks of this conference.
Target audience:
- CEOs, CROs, Directors Managers and Researchers associates Academic professionals
- Industrial expert and scientists
- Regulatory and clinical Scientists
- Researchers, Education providers
- Medicine Deans, Principals, Heads, Professors and Lecturers
- Students and postdoctoral fellows
- Pharmacists, Medical Representatives
- Government Agencies, Medical Directors
- Medical Officers and Executives
- Medical Practitioners
- Clinical pharmacologists
- Clinical Toxicologists
- Molecular and Cellular Pharmacologists
- Pharma Students, PHDs and PDFs
- Medicine Company Directors, MD, Executives, Sales Representatives
Industry Overview and Competitiveness:
Largely, expanded and worldwide, the U.S. pharmaceutical industry is a standout amongst the most basic and aggressive divisions in the economy. As indicated by the Pharmaceutical Research and Manufacturers Association (PhRMA), in excess of 810,000 individuals work in the biopharmaceutical business in the United States over a wide scope of occupations, for example, logical research, specialized help and assembling.
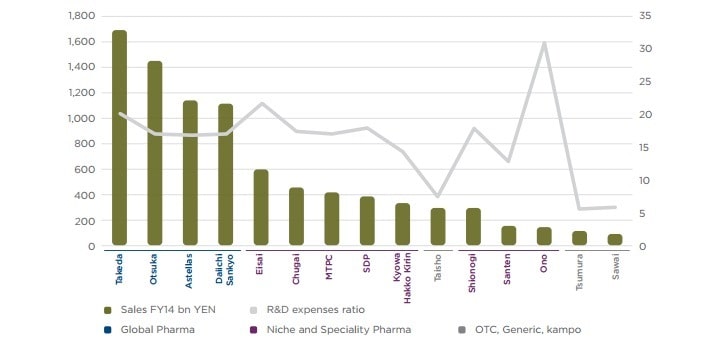
Specifically and in a roundabout way, the industry bolsters more than 3.4 million employments over the United States and added an expected $790 billion to the economy in 2014. The worldwide market for pharmaceuticals is projected to grow from around $1 trillion in 2015 to $1.3 trillion by 2020, representing an annual growth rate of 4.9 percent. Several The worldwide markets for pharmaceuticals are projected to grow from around $1 trillion in 2015 to $1.3 trillion by 2020, representing an annual growth rate of 4.9 per cent.
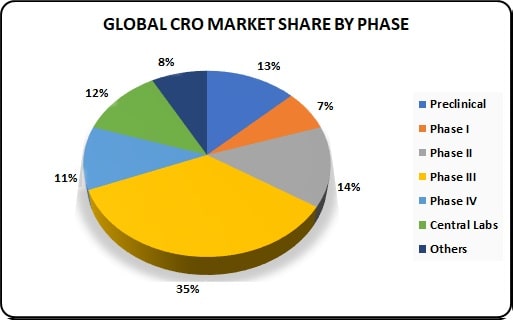
Industries Associated with Pharmaceutical Technology and Development:-
- International Society of Pharmaceutical Compounding, USA
- International Young Pharmacists' Group, USA
- Parenteral Drug Association, Japan
- Regulatory Affairs Professionals Society, USA
- Al-Hayat Pharmaceuticals, USA
- Amgen, Europe
- Elis Pharmaceuticals Limited, Japan
- Alliance Global, Japan
- Sanofi Aventis, UAE
- Genpharm, UAE
- Ranbaxy Laboratories Limited, USA
- BioPharma, Japan
Major Pharmaceutical Associations around the Globe
- Bio analytical Focus Group and Ligand Binding Assay, USA
- American Association of Pharmaceutical Scientists, USA
- Royal Netherlands Chemical Societ, Netherlands
- The European Bio analysis Forum, Europe
- BEBAC Consultancy Services for Bioequivalence and Bioavailabilit, Australia
- European Federation of Pharmaceutical Industries and Associations, Europe
- European Generics and Bio similar Medicines Association, Europe
- Canadian Generic Pharmaceutical Association, Canada
- Bioequivalence and Bioavailability forum, Brazil
- Generic Pharmaceutical Association, USA
- FDA Office of Surveillance and Epidemiology, USA
- ORPHANET Parenteral Drug Association, USA
- PharmGKB, USA
- US Food and Drug Administration , USA
- American Association for Clinical Chemistry, USA
- American Association of Pharmaceutical Scientists, USA
- Clinical Trials Information from National Institutes for Health, USA
- National Institute of Standards and Technology, USA
- Association of the British Pharmaceutical Industry, UK
- Pharmaceutical Research and Manufacturers of America, USA
- Medicines and Healthcare products Regulatory Agency, UK
- Innovative Medicines Canada, CANADA
- Advances in BA&BE
- BA/BE of Biologics & Biosimilars
- Pharmacodynamics
- Pharmacovigilance
- Biopharmaceutics Applications & Clinical Pharmacology
- Pharmacology: PK & PD approach
- Public Health and Nutrition
- Regulatory Requirement and Approaches
- New Approaches to Beat Multi-Drug Resistance: Natural and QSAR-designed Antimicrobial Peptide
- Bioequivalence Protocol: In-Vitro / In-Vivo Studies
- Journal of Pharmaceutical Sciences & Emerging Drugs
- Journal of Pharmaceutics & Drug Delivery Research






















































































































